Luis Espinoza1, Caroline Perez2, Diego Bueno2 and Maria Jose Miguez-Burbano3
1University of Miami Miller School of Medicine, Miami, FL, USA
2Florida International University Modesto Maidique Campus, Miami, FL, USA
3Florida International University/School of Integrated Sciences and Humanity, Miami, FL, USA
- *Corresponding Author:
- Miguez-Burbano MJ
Florida International University/School of Integrated Sciences and Humanity
Miami, FL, USA
Tel: 7864273054
E-mail: mjmiguez@fiu.edu
Received date: August 02, 2016; Accepted date: September 03, 2016; Published date: September 08, 2016
Citation: Espinoza L, Perez C, Bueno D, et al. A Bidirectional Relationship between Smoking and HIV in the Era of Antiretroviral Therapy (ART). 2016; 2:3.
Copyright: © 2016, Espinoza L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Smoking; HIV/AIDS; ART; Cotinine; Viral load
Introduction
While substantial progress has been made in reducing the
prevalence of smoking to approximately 20% among the
general population, these statistics are still grimmer for People
living with HIV (PLWH) in the US [1]. The smoking rates among
this population are two to four times higher (40-80%),
increasing the risks of numerous diseases and threatening
health gains achieved with antiretroviral treatment (ART) [1].
These days PLWH receiving ART lose more life-years to
smoking than to HIV, with mortality tripling compared to the
general population [1,2].
Examinations of the relationship between cigarette smoking
and course of the HIV disease have rendered inconsistent
findings. Many articles suggested that smoking may not impact
HIV disease, T cell counts, or viral load [3]. Yet those findings
need to be considered with the caveat that they were done
near the beginning of the HIV epidemic. More recently, our
group, along with others, started questioning such results
because the immune response associated with tobacco related
diseases should exacerbate viral replication [4]. In support
with our initial paper and postulates, a recent study, the
Women’s Health Initiative and the Ryan White Part A services
in the New York Eligible Metropolitan Area, found a strong
association between smoking and the effectiveness of
treatment [5].
While several of those studies examined the effect of
tobacco on ART and HIV, to our knowledge no prior studies
have examined the impact of ART on nicotine. Yet the nicotine
present in tobacco is predominantly metabolized by hepatic
cytochrome P450 2A6 (CYP2A6) and CYP3A4, which
metabolizes approximately half of the commercially available
drugs including ART [4]. The proposed analyses is also critical
in light of studies indicating that, for an unknown reason,
smoking cessation treatments seem to be less successful
among PLWH. We therefore conducted a study to analyse this
bi-directional relationship.
Methods
Study population
Participants were enrolled into one of four groups based on
smoking and HIV sero-status (HIV+ smoker, HIV+ non-smoker,
HIV- smoker and HIV- non-smoker). This cohort study consists
of 200 PLWH and 200 PLWOH smokers and non-smokers
chosen to represent relatively “pure” smokers with minimal
drug use, and without major confounding factors. Subjects
were ineligible if they possessed a significant history of
medical and immunological illnesses (that is liver cirrhosis,
myopathies, malignancies and congenital or acquired
immunosuppressive conditions, such as recipients of
transplants, corticoids and autoimmune diseases). Older
individuals (>50 years) were excluded to avoid the effects of
immune-senescence. The study was approved by Florida
International University’s and the University of Miami School
of Medicine’s Committee for the Protection of the Rights of
Human Subjects. All subjects signed both written informed
consent and HIPAA forms.
The 15% that were lost to follow-up, and those with missing
data, were excluded from the analyses.
Participants assessment protocol
Trained interviewers conducted in-person, computer based
interviews that were followed by a brief physical exam and
collection of blood specimen.
Smoking
The Fagerström Test for Nicotine Dependence is an
extensively used instrument that provides a wealth of
information regarding the quantity of cigarette consumption,
the compulsion to use, and dependence [6]. Type of tobacco
used (cigarettes, length, mentholated or not, use of filters,
cigars, pipes, etc.) was also collected.
We followed the National Health Interview Survey (NHIS)
guidelines to classify a subject as current smoker or a nonsmoker
[7]. In order to control for bias in participants’ reports,
nicotine plasma levels were used to confirm tobacco use.
Biological confirmation
Cotinine concentrations were quantitatively determined by
using the solid phase, competitive ELISA BK kits. Inc. (San
Diego, CA, US). Intensity of color is inversely proportional to the concentration of cotinine in the samples. The cotinine
concentrations were expressed in ng/mL.
HIV outcomes
Our HIV outcomes were biomarkers of HIV disease
progression, which included unsuppressed viral load (viral load
>200 copies/mL) and low CD4 cell count (<200 cells/mm3). To
achieve this goal blood was drawn and flow cytometry was
used to obtain T lymphocyte phenotypic analysis. Viral load
was quantified using the ultrasensitive Amplicor HIV monitor
test (Roche Diagnostic System). The lower threshold for this kit
detection is 20 copies/ml, with a reported linear range of 20–
10,000,000 cp/ml. Virological success was defined as achieving
undetectable VLs.
Treatment and adherence
Following national protocols, the usual ART regimen
combines three or more different drugs, such as two
nucleoside reverse transcriptase inhibitors (NRTIs) and a
protease inhibitor (PI), two NRTIs and a non-nucleoside
reverse transcriptase inhibitor (NNRTI), or other such
combinations.
An AIDS Clinical Trial Group (ACTG, self-reported adherence
questionnaire was used to calculate the percentage of
adherence and triangulated the information with both
pharmacy and medical records [8]. If discordant, we endorsed
medical/pharmacy reports.
Covariates
Structured questionnaires were used to obtain information
on variables that could impact our analyses including sociodemographics.
Gender (male vs. female or transgender) was
obtained and information regarding menstrual cycles and oral
contraceptives was obtained. Other variables deemed
important were adherence, body mass index, dietary intakes,
drug and alcohol abuse.
Results
Table 1 shows the descriptive characteristics in the total
sample, including smoking status. No significant differences
were found across the groups at baseline in two
measurements of social inequalities, education and income.
Participants were on average 39.8 years old; the majority of
participants were African American or Hispanic.
|
Smokers |
Non-Smoker |
P value |
| Age (years) |
40.4 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 8.2 |
38.7 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 9.6 |
0.06 |
| Gender |
|
|
|
| Male |
61% |
55% |
0.37 |
| Female |
39% |
45% |
|
| Annual Income |
|
|
|
| <$20,000 |
88% |
82% |
|
| ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâà$20,000-$49,999 |
10% |
14% |
0.3 |
| >$49,999 |
2% |
4% |
|
| Education (years) |
11.3 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 2.3 |
11.8 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 2.3 |
0.06 |
| Body Mass Index |
29.7 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 7.4 |
29.8 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 6.9 |
0.92 |
| Albumin (mg/dl) |
4.3 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 0.3 |
4.2 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 0.3 |
0.22 |
| SGOT (IU/L) |
32.2 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 13 |
33 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 17 |
0.85 |
| SGPT (IU/L) |
32.6 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 18 |
35.9 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 29.6 |
0.39 |
Table 1: Sample characteristics and smoking status (Mean values (SD) for continuous variables and n (%) for categorical variables.
Except for age and education, the groups were very similar. Please note that even in these cases groups differed only by 2 years).
Smoking and HIV outcomes
As seen in Table 2, subjects smoked as little as 1 cigarette
per day and as much as 4.5 packs. The average level of
cumulative tobacco exposure was 10 pack-years; with our
typical participant was a smoker, on average, for half of their
life (approximately two decades).
The mean CD4 cell counts (cells/μL) tended to differ
between smokers and non-smokers (317.3 ± 43) and HIV positive smokers (260.6 ± 24). Despite similar medication
adherence (90% vs. 80%), there was a significant increase in
the viral burden in smokers compared with non-smokers
(37179.0 ± 10121.0 vs. 10797.5 ± 3123 copies). Additional
analyses indicated that smokers were two times more likely to
fail at achieving undetectable viral loads (OR=1.4; 95% CI 1-2.1,
p=0.04).
|
Smokers |
Non-Smoker |
P value |
| Age (years) |
40.4 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 8.2 |
38.7 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 9.6 |
0.06 |
| Gender |
|
|
|
| ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàMale |
61% |
55% |
0.37 |
| ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàFemale |
39% |
45% |
|
| Annual Income |
|
|
|
| <$20,000 |
88% |
82% |
|
| ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡ÃâàÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâà$20,000-$49,999 |
10% |
14% |
0.3 |
| >$49,999 |
2% |
4% |
|
| Education (years) |
11.3 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 2.3 |
11.8 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 2.3 |
0.06 |
| Body Mass Index |
29.7 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 7.4 |
29.8 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 6.9 |
0.92 |
| Albumin (mg/dl) |
4.3 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 0.3 |
4.2 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 0.3 |
0.22 |
| SGOT (IU/L) |
32.2 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 13 |
33 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 17 |
0.85 |
| SGPT (IU/L) |
32.6 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ 18 |
35.9 ÃÆÃââââ¬Ã
¡ÃÆââ¬Å¡Ãâñ29.6 |
0.39 |
Table 2: Tobacco outcomes. Mean values (SD) for continuous variables and n (%) for categorical variables. Despite smoking similar
number of cigarettes per day and start smoking at similar age, HIV positive individuals exhibited significantly higher levels of
cotinine.
HIV, antiretroviral treatment and biomarkers of
smoking
Our analyses revealed that HIV positives and negatives
smoked similar quantities per day (7.5 ± 0.6 Vs 7.8 ±8.3
cigarettes/day). Yet, the circulating levels of cotinine were
slightly higher in HIV positives compared to their seronegative
counterparts (224.4 ± 198 vs. 190.8 ± 164 ng/ml, p=0.09).
After multivariate adjustment, cotinine levels were an average
of 40 ng/ml higher among PLWH receiving ART than those
without it (p=0.05).
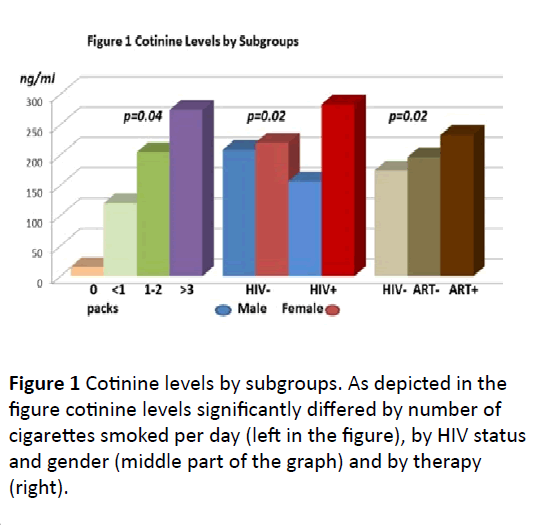
Female smokers had slightly higher serum cotinine
concentrations than the males (220.8 ± 22 versus 208 ± 15
ng/ml, p=0.6). However, while PLWOH’s cotinine levels were lower in females than in males, HIV-infected females had
significantly higher cotinine levels than HIV infected males.
Indeed, despite smoking similar amounts (males=8.0 ± 0.7 vs.
6.9 ± 0.9 cigarettes/day) cotinine values in HIV-infected
females almost doubled compared to their non HIV-infected
counterparts (283 ± 36 vs. 155.7 ± 19 ng/ml, p=0.01). Since
prior studies have evidence that hormonal changes can affect
nicotine metabolism we proceeded with analyses regarding
their menstrual cycles [9]. In the descriptive analyses 19% of
females had irregular menstrual cycles, 7% had been
submitted to hysterectomy, and the remaining females (74%)
reported normal menstrual cycles. Among HIV-infected
females, those with irregular menstrual cycles exhibited the
highest cotinine levels (410 ± 85 vs. 202 ± 32 ng/ml, p=0.02). However, such a relationship did not hold in female PLWOH
(Figure 1 and Table 3).
|
Coefficients |
|
|
| Model |
Unstandardized Coefficients |
Standardized Coefficients |
t |
Sig. |
|
B |
Std. Error |
Beta |
|
|
| (Constant) |
219.064 |
164.9 |
|
1.328 |
0.19 |
| Antiretroviral |
80.426 |
39.192 |
0.266 |
2.052 |
0.05 |
| Menopause/irregular cycles |
-162.99 |
70.511 |
-0.3 |
-2.31 |
0.03 |
| CD4 Absolute Counts |
-0.01 |
0.022 |
-0.056 |
-0.44 |
0.66 |
| Packs |
119.028 |
50.055 |
0.304 |
2.378 |
0.02 |
Table 3: Regression analyses; predictors of cotinine levels (a: Dependent Variable: Cotinine), Model 1: Adjusted for age (years) alcohol consumption, body mass index (normal weight/overweight/obese), physical activity (inactive/low/medium or high); viral load. A pack-year was defined as having smoked 20 cigarettes per day.
Figure 1: Cotinine levels by subgroups. As depicted in the
figure cotinine levels significantly differed by number of
cigarettes smoked per day (left in the figure), by HIV status
and gender (middle part of the graph) and by therapy
(right).
Discussion
In accord with prior in vitro and in vivo studies, our data
demonstrated that smoking is associated with increased viral
burdens and poor viral responses among those receiving
antiretroviral therapy [9-12]. Our analyses expanded the
literature in the field by demonstrating that after controlling
for the amount and type of cigarettes smoked, HIV infected
smokers had higher cotinine levels than non-HIV infected ones.
This is the first clinical evidence of a bi-directional relationship.
Differences in cotinine levels between seropositives and
seronegatives are consistent with prior studies signifying that
these at-risk populations experience more difficulty quitting
[13]. Our study provides further information by demonstrating
that antiretroviral therapy may be driving at least part of the
increased levels of cotinine observed in this population.
Findings were not unexpected when considering that tobacco
is metabolized by hepatic cytochrome P4502A6 (CYP2A6) and
CYP3A4 which metabolizes many antiretroviral. More
specifically CYP is involved in the metabolism of protease
inhibitors and non-nucleoside reverse transcriptase inhibitors
[10]. Findings are clinically relevant as they highlight the need
for Nicotine replacement therapy dose adjustments during
pharmacological interventions to temper/match nicotine
intake prior to quitting. The observed increases are also of
concern because it can lead to increases in the production of
reactive oxygen species oxidative stress and organ damage.
Notably, data also indicated that the association between
HIV/ART and cotinine levels was stronger and statistically
significant for women. Analyses have shown that gender
differences in cotinine levels are not related to a greater
number of cigarettes smoked, rather it is probably due to
hormone driving differences [14]. Indeed, higher levels were
evident among those self-reporting amenorrhea, changes in
menstrual cycles, early menopause, or hysterectomy. Findings
are of great concern in light of studies indicating that the
health consequences of tobacco use are greater among female
smokers [15]. Equally important, our results can partially
explain prior observations indicating less favourable results
among women trying to quit, which could reflect the need for
higher NRT doses. Results also highlight the need of additional
research and tailoring therapies, because the “one size fits all”
approach clearly does not work for this study population.
This study has some limitations. First, the population was
limited to those living in South Florida. Although we had
dietary data, we cannot analyse differences in intakes of some
dietary products such as tea, tomatoes, eggplants, and
potatoes that have nicotine, but these should contribute
minimal amounts.
In summary the data indicated the impact of HIV and
antiretroviral therapy on cotinine levels and provided a
potential explanation for why people living with HIV have
additional difficulties quitting. The analyses highlight the
impact of gender when studying behaviours and treatment outcomes, in order to provide relevant and useful information
to clinicians and health policymakers.
Acknowledgement
People who contributed to the work, but do not fit the
criteria for authors should be listed in the Acknowledgments,
along with their contributions. You must also ensure that
anyone named in the acknowledgments agrees to being so
named.
Funding
This study was funded by the James and Esther King
Biomedical Research Program, Florida Health Department
(DOH/1KG10-3398).
Competing Interests
Authors received salary support from the James and Esther
King Biomedical Research Program, Florida Health Department
for the submitted work. There are no other relationships or
activities that could influence the submitted work.
References
- Kariuki W, Manuel JI, Kariuki N (2016) HIV and smoking: Associated risks and prevention strategies. HIV/AIDS 8: 17-36.
- Lifson AR, Neuhaus J, Arribas JR (2010) Smoking-related health risks among persons with HIV in the strategies for management of antiretroviral therapy clinical trial. Am J Public Health 100: 189-1903.
- Kabali C, Cheng DM, Brooks DR (2011) Recent cigarette smoking and HIV disease progression: No evidence of an association. AIDS Care 23: 947-956.
- Ande A, McArthur C, Kumar A(2013) Tobacco smoking effect on HIV-1 pathogenesis: Role of cytochrome P450 isozymes. Expert Opinion on Drug Metabolism and Toxicology 9: 1453-1464.
- Health Resources and Service Administration HIV/AIDS Programs (2009) Ryan White HIV/AIDS Program Part A- Grants to Emerging Metropolitan and Transitional Grant Areas.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991). The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance tuestionnaire. Br J Addict 86: 1119-1127.
- Centers for Disease Control and Prevention (2016) National Health Interview Survey.
- Chesney MA, Ickovics JR, Chambers DB (2000) Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee and Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care 12: 255-66.
- Royce RA, Winkelstein W Jr (1990) HIV infection, cigarette smoking and CD4+ T-lymphocyte counts: Preliminary results from the San Francisco Men's Health Study. AIDS 4: 327-333.
- Kumar S, Jin M, Ande A (2012) Alcohol consumption effect on antiretroviral therapy and HIV-1 pathogenesis: Role of cytochrome P450 isozymes. Expert Opinion on Drug Metabolism andToxicology 8: 1363-1375.
- Abbud RA, Finegan CK, Guay LA (1995) Enhanced production of human immunodeficiency virus type 1 by in vitro-infected alveolar macrophages from otherwise healthy cigarette smokers. J Infect Dis 172: 859-863.
- Zhao L, Li F, Zhang Y (2010) Mechanisms and genes involved in enhancement of HIV infectivity by tobacco smoke. Toxicology 278: 242-248.
- Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, et al. (2000) Human immunodeficiency virus infection, aids and smoking cessation: The time is now. Clin Infect Dis 31: 808-812.
- Carpenter MJ, Upadhyaya HP, LaRowe SD (2006) Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: A review. Nicotine Tob Res 8: 627-638.
- CDC (2016) Women and smoking.