Research Article - (2017) Volume 3, Issue 1
Quintero Pulido DF1*, Ten Kortenaar MV2, Hurink JL1 and Smit GJM1
1Electrical Engineering, Mathematics and Computer Science, University of Twente, Enschede, The Netherlands
2Dr Ten BV, Rondweg 11M/N, 8091 XA Wezep, The Netherlands
Corresponding Author:
Quintero Pulido
Computer Science, Department of Electrical Engineering
Mathematics and Computer Science
University of Twente, P.O. Box 217
7500 AE Enschede, The Netherlands
Tel: 0031621323089
E-mail: d.f.quinteropulido@utwente.nl
Received Date: June 07, 2017; Accepted Date: June 27, 2017; Published Date: July 03, 2017
Citation: Pulido DFQ, Kortenaar MVT, Hurink JL, et al. A Practical Approach in Glycerol Oxidation for the Development of A Glycerol Fuel Cell. Trends Green Chem. 2017, 3:1. doi: 10.21767/2471-9889.100018
The research on electrochemical carbon molecule oxidation started in the past years as new electrochemistries were researched for new fuel cells systems and batteries that may line up as backup energy supply and storage systems in off-grid and on-grid microgrids, as modeled by our group at the University of Twente [1-3]. By lining up these two disciplines we hope to support the bridge between new electrochemical systems on one side, (pilot) production with partner companies, prediction, and validation of systems upon implementation in microgrids by our group.
The electrochemical oxidation of glycerol in alkaline aqueous solution has been studied on gold and gold coated metals (Zn-Au and Cu-Au) by voltammetry and EIS (Electrochemical impedance spectroscopy) for possible use in a new fuel cell as an outlet for the excess glycerol that is produced in the biodiesel industry. The observations show that the gold surface may change upon cycling by cyclic voltammetry. Besides, the current density shows non-linear behavior with the square root of the scan rate, implying that the reaction is not totally controlled by diffusion. EIS analysis using the EQUIVCRT software revealed that one out of twenty tested equivalent circuits fitted the data well at potentials of -0.05 V,- 0.15 V and -0.25 V vs. Ag/AgCl, identifying resistors and a Warburg element in parallel with the double layer capacitance, the elements are possibly related to the presence of double layers associated with hydroxypyrovate and oxalate ions. The results are consistent with the low-frequency error fitting analysis (10-4), AC Simulink-Matlab fitting responds and the Kronig-Kramers transform test. The tested Zn-Au and Cu-Au electrodes show similar voltammetry behavior as the gold electrode, as witnessed by the results of cycle analysis and the scan rate analysis. The discharge chronoamperometry test further shows that the Zn-Au electrode and Cu-Au have higher current densities than the gold electrode at a potential of -0.25 V vs. Ag/AgCl (5 mA cm-2, 4.5 mA cm-2, and 3 mA cm-2 respectively).
Keywords
Microgrids; Voltammetry; Oxidation; Gold; Glycerol; Impedance
Introduction
The growing amount of biodiesel produced worldwide leads to more than two million tons of glycerol entering the market yearly [4]. Glycerol is used in the pharmaceuticals, cosmetics and food industries. However, the current production rate already surpasses the capacities needed by these industries. The countries with high production of biodiesel (e.g. USA, Germany and Colombia) are facing serious challenges with the glycerol overproduction changing the value of glycerol dramatically [5]. This specific situation has led to consistently generated low glycerol prices, making glycerol a bio-waste product in the need for new market alternatives. One alternative to valorise glycerol might come from electrochemical oxidation which may give way to oxygenated materials with higher market value (tartronic acid, dihydroxyacetone, and glycolic acid, among others) [6]. The electrochemical oxidation of glycerol may be performed using a fuel cell. Fuel cells can convert directly a fuel into electricity [7]. One common fuel use in fuel cells is H2 and its production is well known in chemical production plants. However, to store hydrogen is still a challenge due to its high volatility and safety concerns [8], one alternative to the problem is to use a fuel that is liquid under ambient conditions. There are different types of fuels that were tested in the past as possible alternative fuels such as, hydrazine, organic compounds, and formic acid [9-11]. Nevertheless, there are technical and commercial necessities that a fuel must comply in order to be used in a fuel cell e.g. availability, transport, safety, and cost. Taking this into account, the quantity of possible fuels is reduced to a few types.
Electrochemical oxidation of glycerol has already been considered in the past, and special attention was given towards the selection of catalyst in alkaline and acid media. The following materials have been reported: Pt [12], Pt-Ru [13], Au, Pd [14,15] and Ag [16]. In the case of gold there are several advantages over other catalyst used for glycerol oxidation, e.g. it has a fast electrocatalytic response, is stable in alkaline media, is more abundant and it has a lower price than palladium or platinum [17]. Although gold has been studied as a catalyst for glycerol oxidation, the mechanism of the reaction is still far from being understood. E.g. in the study of Ureta [18], the mechanism of alcohol oxidation in alkaline media on a gold electrode was investigated and it is suggested that the optimal oxidation for alcohols is at pH 11. Also, Shi [19] studied the kinetics of low concentration of glycerol in alkaline media using voltammetry on a gold foil. Recently, Qi [20] studied the generation of tartronade during glycerol oxidation in alkaline media, using an AuC coated electrode. From these studies were concluded that glycerol oxidation reaction at gold electrode begins with two primary -OH groups and the chemical reaction possibly decrease the overoxidation of the secondary -OH and C-C bond cleavage. Also, Chornaja [21] proved that the glycerol oxidation pathways are probably two depending on the studied catalyst, leading to the formation of intermediate compounds such as tartronic, lactic, glycolic and oxalic ions. Marshall [22], studied the influence of gold nanoparticles loading on Au/C electrode towards glycerol oxidation showing that the oxidation process is dependent on particle load and size distribution. Kwon [23,24] studied the mechanism of glycerol oxidation on polycrystalline gold and platinum electrodes, Kwon found that the glycerol oxidation is strongly influenced by pH, a no catalytic activity was observed in acid media. Padayachee [25] used electrochemical impedance spectroscopy (EIS) with an Au/MnO2/Carbon alloy electrode to compare resistances at different potentials. In his study was determined that the lowest cell resistance is at values of 0 V to 0.2 V vs. Ag/AgCl implying an optimal potential for glycerol oxidation. However, no clear conclusion was drawn for the circuit fitted for the reaction.
In order to build a glycerol oxidation technology viable like in a fuel cell, the mechanism of the chemical reaction has to be studied in more detail to understand the reaction pathways and the kinetics. Also, to make this approach feasible, still more studies need to be done towards development and research of stable electrodes with low catalyst load.
This research aims to yield to an approach for a possible development of a glycerol fuel cell by observing the glycerol oxidation mechanism and kinetics using a gold electrode in alkaline media. Besides, it aims to compare the electrochemical behavior of gold coated metals prepared in situ (Zn-Au and Cu- Au) in order to reduce the gold load used for glycerol oxidation and for practical implementation. The practical implementation is related to the use of the glycerol fuel in microgrids. The simulation of renewable energies (solar panel, wind turbines to name a few) combine with batteries and fuel cell is under simulation in our group in The Netherlands [3], however, the large-scale application goes along with an understanding of the chemical, electrochemical and physical characteristics of the different technologies.
This paper is organized as follows: Experimental methods and the EIS fitting circuit is explained. General approach used in this paper for data interpretation is presented. Experimental results testing the gold electrode is divided in three parts. First, voltammetry is used for comparison of catalyst for glycerol oxidation (Pt, Ag, Au, Cu and glassy carbon). Second, the cyclic voltammetry analysis of cycles and scan rate effect of glycerol oxidation in alkaline aqueous solution on gold electrode is shown. And third, the EIS results for the study of the mechanism that the glycerol oxidation reaction undertakes when using the gold electrode. Practical test with the gold coated Zn and Cu electrodes analyzed with cyclic voltammetry and chronoamperometry (cycles and scan rate effect) to observe the glycerol oxidation in alkaline media in comparison with the gold electrode. Lastly, the conclusions are presented.
Experimental Methods
The reagents: Glycerol (C3H8O3) ≥ 98%, H2SO4 ACS reagent, 37%, AuBr2 reagent ≥ 99% and NaOH reagent grade ≥ 98%, pellets were purchased from Sigma-Aldrich were used with no further purification.
The equipment: Cyclic voltammetry-chronoamperometry experiments were performed with a PGSTAT 101 compact unit from the company Metrohm Autolab. The pH and temperature were recorded with a Hanna HI 9811-5 pH meter. Impedance spectroscopy was performed with a Model 600E Series Electrochemical Analyzer from the company CH instruments.
The EIS analysis: EIS analysis was done with the EIS fitting procedure in which the elements are analyzed with the software method EQUIVCRT developed by Boukamp [26-28]. This method provides information on the kinetics of the reaction and are validated by a linear Kronig-Kramers transform test method developed by the same author [29]. In this paper, a circuit fitting test in Simulink-Matlab for a view of AC signal output is used, in order to validate the circuit fitted.
The Electrochemical set up: A three electrode ensemble was used for this study. Various working electrodes were used: Glassy carbon (GC), Au, Ag and Pt (Autolab electrodes) and metals such as Zn, Cu and carbon graphite (CG) 98% grade materials from Sigma-Aldrich. The counter electrode was carbon graphite and the reference electrode Ag/AgCl. The electrode ensemble was immersed in a 50 ml aqueous electrolyte.
Electrochemical coating: Glassy carbon, Zn, and Cu (area 0.072 cm2) were immersed first in a solution of 1 M H2SO4 for a few seconds to remove impurities, then rinsed with demi-water and dried for an hour. Each of the electrodes was immersed separately in an electrochemical cell with 0.01 M AuBr2 as the electrolyte. AuBr2 was selected for electroplating as the subproducts of the electrodeposition are more environmentally friendly than common gold cyanate electrodeposition [30]. The cathode material used was carbon graphite and no reference electrode was used. Then, a potential of 2.0 V was fixed (letting the current free) in the cell for 30 s in order to develop a layer of gold as witnessed by a change in color on the electrode. During the experiment, any smell of bromine was not detected. After the electrode plating method, the electrodes were rinsed with demi-water and dried during the night.
General Approach
An ideal fuel cell system may be interpreted by means of cyclic voltammetry, taking anode and cathode reactions separated. If the two reactions are combined in one plot they may be depicted as shown in Figure 1. In a good glycerol fuel cell, glycerol is oxidized at the anode, and the current density, therefore, should start to rise at a potential as negative as possible. The value of this potential is generally determined by the catalyst, side reactions, and cell resistance. Meanwhile, at the cathode oxygen is reduced and the current density should start to increase at a potential as positive as possible. The max potential of the system is provided by the difference in the glycerol oxidation and oxygen reduction potentials and the current by the steady state rates of these reactions at the anode and at the cathode respectively. This is possible by taking into account the lowest possible ohmic resistance, which is determined by cell geometry, impurities and reaction kinetics. This research is nevertheless focused on analyzing gold as an anode electrode for glycerol oxidation and its possible use in a fuel cell. As literature showed that gold is a relative good electrode, is less corrosive and stable at different pH. Consequently, we looked for the lowest ohmic resistances and low cost substrates for gold coating.
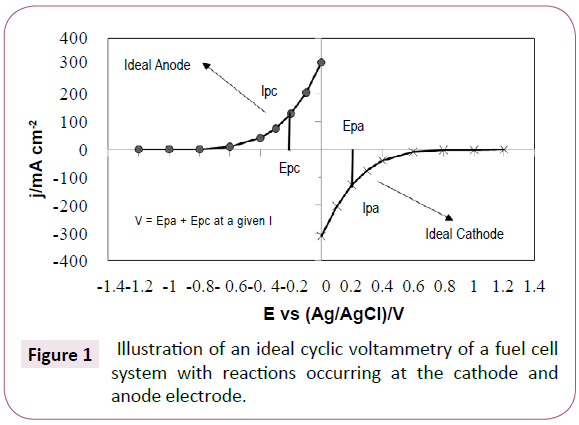
Figure 1: Illustration of an ideal cyclic voltammetry of a fuel cell system with reactions occurring at the cathode and anode electrode.
Results and Discussion
Analysis of catalyst for glycerol oxidation
In order to gain insight into the glycerol oxidation, cyclic voltammetry is used on the different catalyst as shown in Figure 2. The goal was to get a quick comparison of the electrochemistry of different catalyst in the same electrolyte. The measurements were done at 100 mV/s in 1 M glycerol in 1 M NaOH.
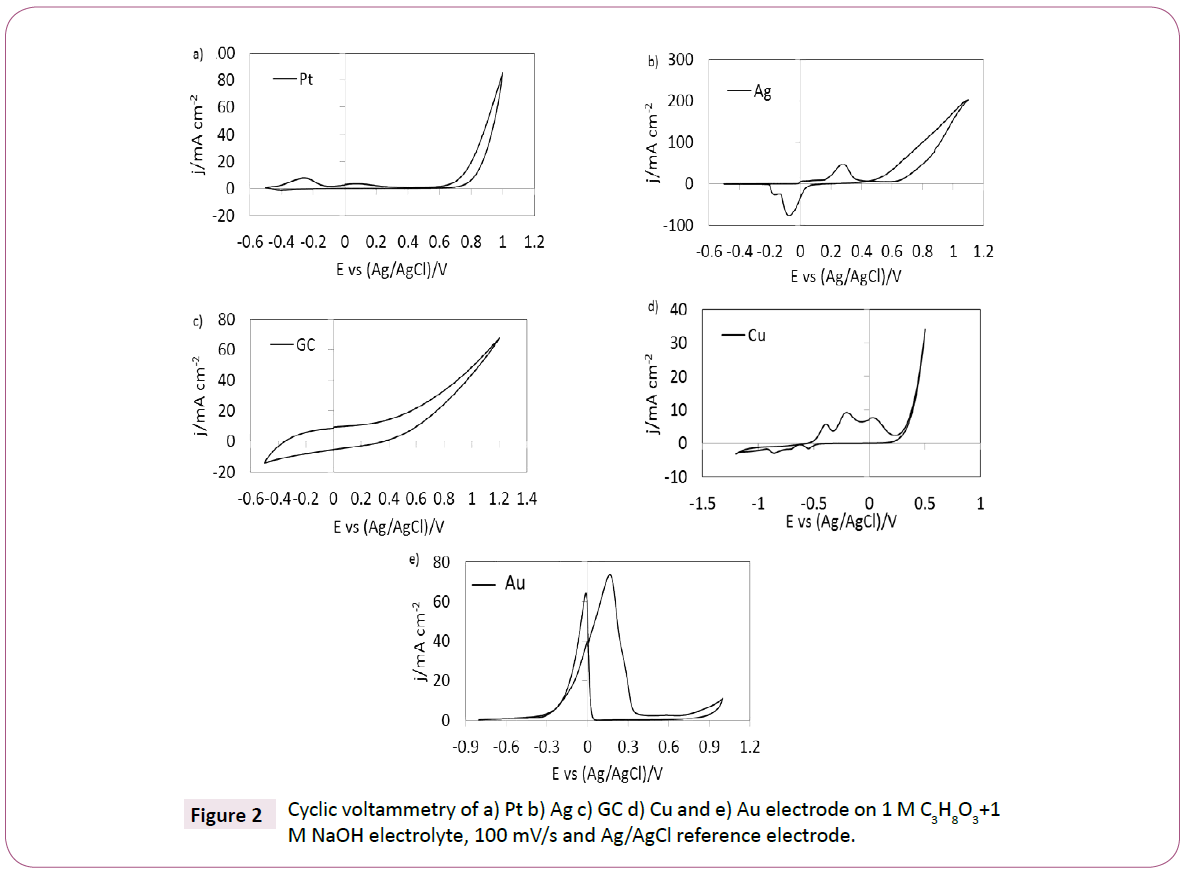
Figure 2: Cyclic voltammetry of a) Pt b) Ag c) GC d) Cu and e) Au electrode on 1 M C3H8O3+1 M NaOH electrolyte, 100 mV/s and Ag/AgCl reference electrode.
The voltammogram for the catalytic effect of Pt for glycerol oxidation in alkaline media in the potential window of -0.6 V to 1 V vs. Ag/AgCl is shown in Figure 2a. The first peak (-0.3 V and 10 mA cm-2) corresponds to Pt reduction and the second peak corresponds to glycerol oxidation (around 0.1 V and 5 mA cm-2) in accordance with research reported by Akinbayowa [13]. Figure 2b and 2c show the voltammetry of a silver and glassy carbon electrode for glycerol oxidation in alkaline media respectively. Clearly, silver and a glassy carbon have less catalytic effect for glycerol oxidation which is in-line with research performed by Song [31]. The voltammetry for the copper show three current density peaks at different potentials at -0.3 V,-0.1 V and 0.1 V vs Ag/AgCl (Figure 2d). The first two current density peaks (from left to right) might correspond to copper oxidation in different states (Cu3+ to Cu and Cu2+ to Cu) as studied by Rozali [32]. The third peak may be interpreted as a glycerol oxidation. However, it may also be related to corrosion as explained in literature [33]. The glycerol oxidation in alkaline aqueous solution on gold is shown in Figure 2e, where two current density peaks at -0.2 V and -0.1 V potential are observed. The first peak corresponds to a typical sudden increase in oxidation upon reducing gold from its oxidized state in alkaline media.
The second peak corresponds to the glycerol oxidation which is presented in the literature by different authors [14-22]. In general, the catalytic activity for glycerol oxidation is observed with Pt, Cu, and Au electrodes. However, for the Ag and GC electrodes, no clear evidence of glycerol oxidation is observed, when comparing the current densities of the different metals at a potential of -0.2 V. The results show that the gold electrode has the highest value (11 mA cm-2) followed by copper (8 mA cm-2) and the platinum electrode has the lowest current density (4 mA cm-2). Based on these observations, we focused on the catalytic activity of gold and gold coated Zn and Cu electrodes for glycerol oxidation, as further reported below.
Au electrode kinetics
Figure 3a shows repeated voltammetry cycling (30 cycles) in the potential window between -0.85 V to 0 V vs. Ag/AgCl at Au electrode in 1 M glycerol +1 M NaOH. After each cycle, an increase in current density is observed. This increase in current density may be related to changes in the surface of the electrode. The change in the surface was observed before and after the cycling experiment in Figure 3b. Each cycle may have an effect on the electrode surface, which could affect the catalytic area for the oxidation reaction, thus increasing the current density during the experiment. The changes in the current density are related also to losses in the faradic activity. Glycerol oxidation is affected by the increase in the surface area, as the glycerol ions approaching the electrode interact with the surface creating larger spaces. The current started to increase at around -0.25 V and the steady increase continues until all glycerol near the surface of the electrode has been oxidized.
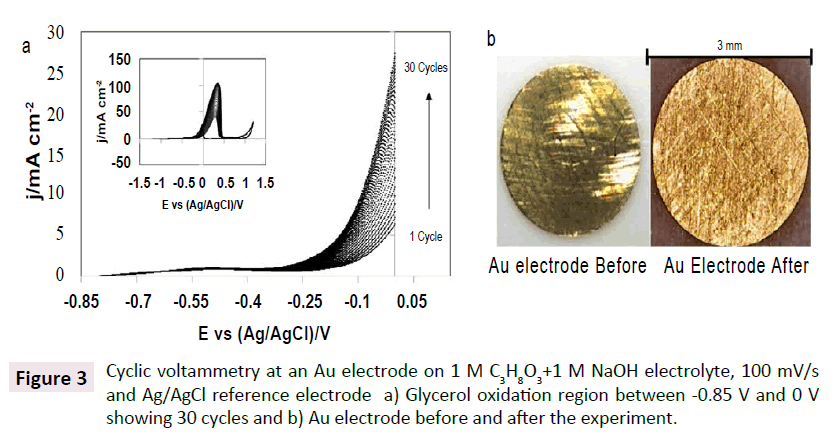
Figure 3: Cyclic voltammetry at an Au electrode on 1 M C3H8O3+1 M NaOH electrolyte, 100 mV/s and Ag/AgCl reference electrode a) Glycerol oxidation region between -0.85 V and 0 V showing 30 cycles and b) Au electrode before and after the experiment.
To understand more about the kinetics of the glycerol oxidation in alkaline media at the gold electrode, the influence of the scan rate is studied in Figure 4a. The current density gradually increased with the scan rates. The correlation between the scan rate and the current density at -0.15 V vs Ag/AgCl was linear at a scan rate coefficient of 1/5 (I vs v1/5) and not at 1/2 a coefficient (Figure 4b). Other scan rate coefficients were tested (Figure S1) but no linearity was observed. This possibly suggests that the glycerol oxidation on gold electrode is not totally controlled by diffusion and does not follow Levich behavior. Also, with the increase of the scan rate the potential was negatively shifted. The varied potential may represent an alteration in the gold surface due to the interaction with glycerol and OH ions [34]. However, no clear evidence was observed in the voltammogram for the formation of oxidase compounds. EIS studies could elucidate details about the mechanism of the glycerol oxidation at the gold electrode and find close related oxidized compounds.
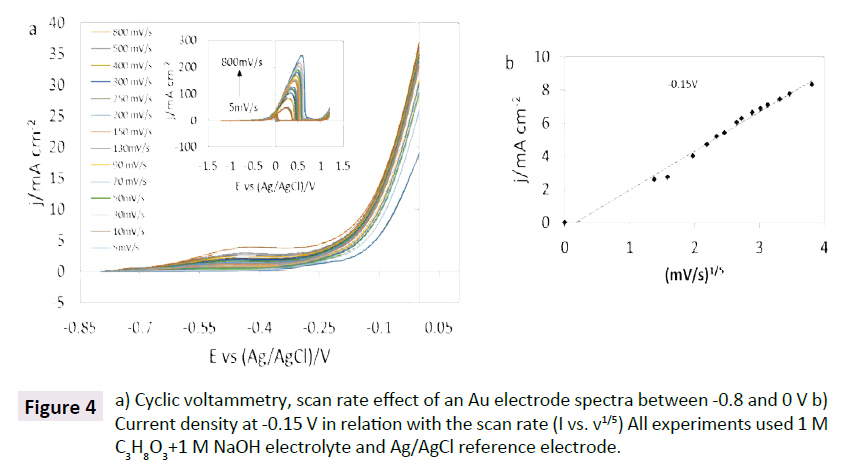
Figure 4: a) Cyclic voltammetry, scan rate effect of an Au electrode spectra between -0.8 and 0 V b) Current density at -0.15 V in relation with the scan rate (I vs. v1/5) All experiments used 1 M C3H8O3+1 M NaOH electrolyte and Ag/AgCl reference electrode.
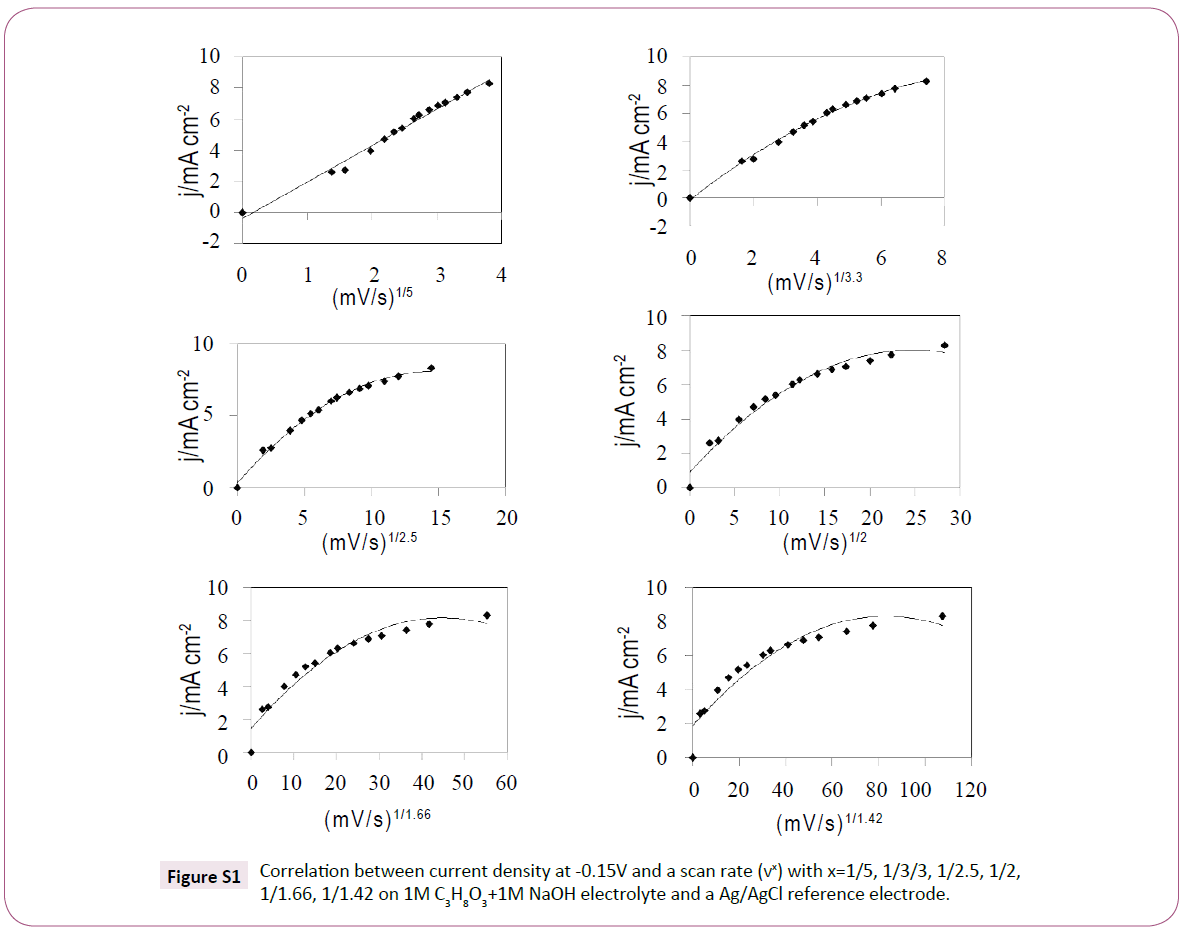
Figure S1: Correlation between current density at -0.15V and a scan rate (vx) with x=1/5, 1/3/3, 1/2.5, 1/2, 1/1.66, 1/1.42 on 1M C3H8O3+1M NaOH electrolyte and a Ag/AgCl reference electrode.
Au electrode mechanism for glycerol oxidation in alkaline media and EIS analysis
EIS analysis was performed in different steps: first, a plausible reaction pathway was taken from literature and a possible reaction model was created. Second, an expected circuit was drawn based on the assumption of the model for the reaction. Third, the circuit was compared with the lab results using the EQUIVCRT software and fourth the fitted circuits were analyzed with the Kramers-kroning transform and AC Simulink-Matlab circuit signal output to validate the data obtained.
Reaction pathways: Wang [12], explained that glycerol oxidation in an alkaline aqueous solution has two pathways depending on the catalyst. The first reaction path was derived for Pt in which, glyceric acid is the main glycerol oxidation product with tartronic, lactic, glycolic and oxalic acids as by-products (Figure 5a). Glycolic and oxalic acids are formed during the oxidation of tartronic acid. In the same study, it was showed that the reaction products of tartronic acid oxidation with dioxygen in the presence of the 5.7% Pt-2.4% Bi/C catalyst are mesoxalic and oxalic acids. However, the formation of glycolic acid was not detected. For the glycerol oxidation on a 1% Au/C catalyst, the authors found that there is another pathway for the reaction in which glycolate and oxalate ions may be created only by the presence of hydroxypyrovate (with the according to intermediate dihydroxyacetone). One plausible explanation for the two glycerol oxidation paths is the behavior of the glycerol molecule in aqueous solution as shown in literature [35]. It is assumed that glycerol oxidation will take place where the electron goes towards the electrode. In aqueous solution, this happens at the electrode-electrolyte interface. This can be considered as a characteristic of a surface phase, in which there is the existence of an electric field, which is caused by the separation of charges that are in the bulk phases in contact, depending on catalyst the rate of the reaction may be different and this can contribute to the different paths observed with the different catalyst.
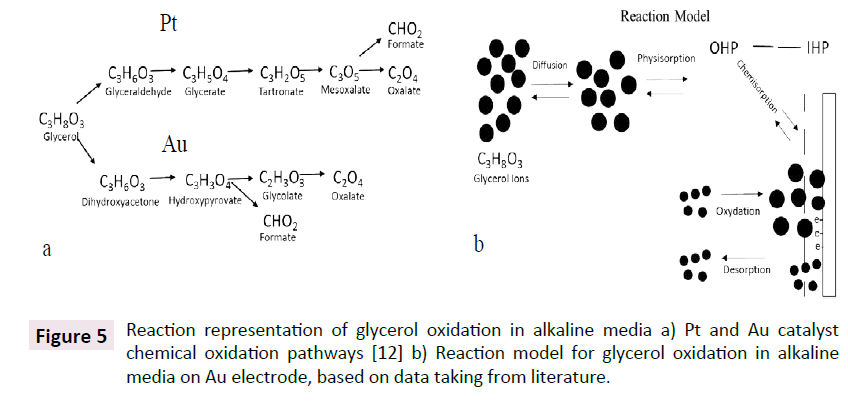
Figure 5: Reaction representation of glycerol oxidation in alkaline media a) Pt and Au catalyst chemical oxidation pathways [12] b) Reaction model for glycerol oxidation in alkaline media on Au electrode, based on data taking from literature.
Expected circuit
By using the glycerol oxidation reaction pathway for gold (Figure 5a), an equivalent circuit was derived. Under the assumption that each reaction step maybe represented by a sub-analog electrical element. First, we considered the chemical reaction that is shown in Figure 5b in which diffusion is followed by physisorption in the outer Helmholtz plane (IHP). Chemisorption processes are presented in parallel to the double layer capacitance in the inner Helmholtz plane (IHP) and various species are captured close to the electrode (oxidation-desorption steps). The concept of a diffusion effect at the beginning of the reaction is related to faradic currents that could occur. This assumption is based on the formation of a thinner Nernst diffusion layer, or with a smaller similar contribution of the absorbed species that are in the overall electrode impedance. The parallel connections in the circuit are associated with the resistances provided by the reactions that occur during physisorption and chemisorption which are related to the oxidation and desorption of ions.
Fitted circuit
The fitting of the circuit was based on the assumption that one particular circuit may fit (or mimic) all data that was found in the impedance measurements. Figure 6a is the circuit expected for EIS fitting from data in literature. The impedance data was collected at three different potentials -0.25 V,-0.15 V and -0.05 V vs Ag/AgCl that are in relation with the voltammetry of gold for glycerol oxidation. After testing over 20 circuits in the software EQUIVCRT developed by Boukamp [26], one circuit seemed to fit well the potentials studied for the glycerol oxidation in alkaline media at the gold electrode (Figure 6b). All spectra had an X2 value below 10-4, but although these X2 values are large, the circuit mimics reasonably the presented data and is in line with our comprehension of the reaction, therefore the fitted results were further analyzed. In principal, some of the resistance and the diffusion element are merged, this might be due to the low influence in the frequency studied or do to extreme low noise in the system. A diffusion element (Warburg) is observed in the circuit in parallel with the double layer capacitance, and it is treated as a CPE (constant phase element (Q)) with a value of n=0.5 with a capacitor and a resistor in parallel.

Figure 6: Equivalent circuits for the glycerol oxidation in alkaline media at an Au electrode a) Circuit expected for EIS fitting from data in literature and b) Circuit fitted with EIS software EQUIVCRT.
Spectra analysis
The impedance plot at different potentials (-0.05 V, -0.15 V and -0.25 V vs Ag/AgCl) was plotted with the real and fitted values, in the voltammetry region for the oxidation of glycerol in alkaline media with the gold electrode are shown in Figure 7a. The lowest resistance is observed in the EIS experiment for the potential of -0.05 V vs. Ag/AgCl which was also related to the largest current in the reaction voltammogram (30 mA cm-2) when compared with the other analyzed potentials.
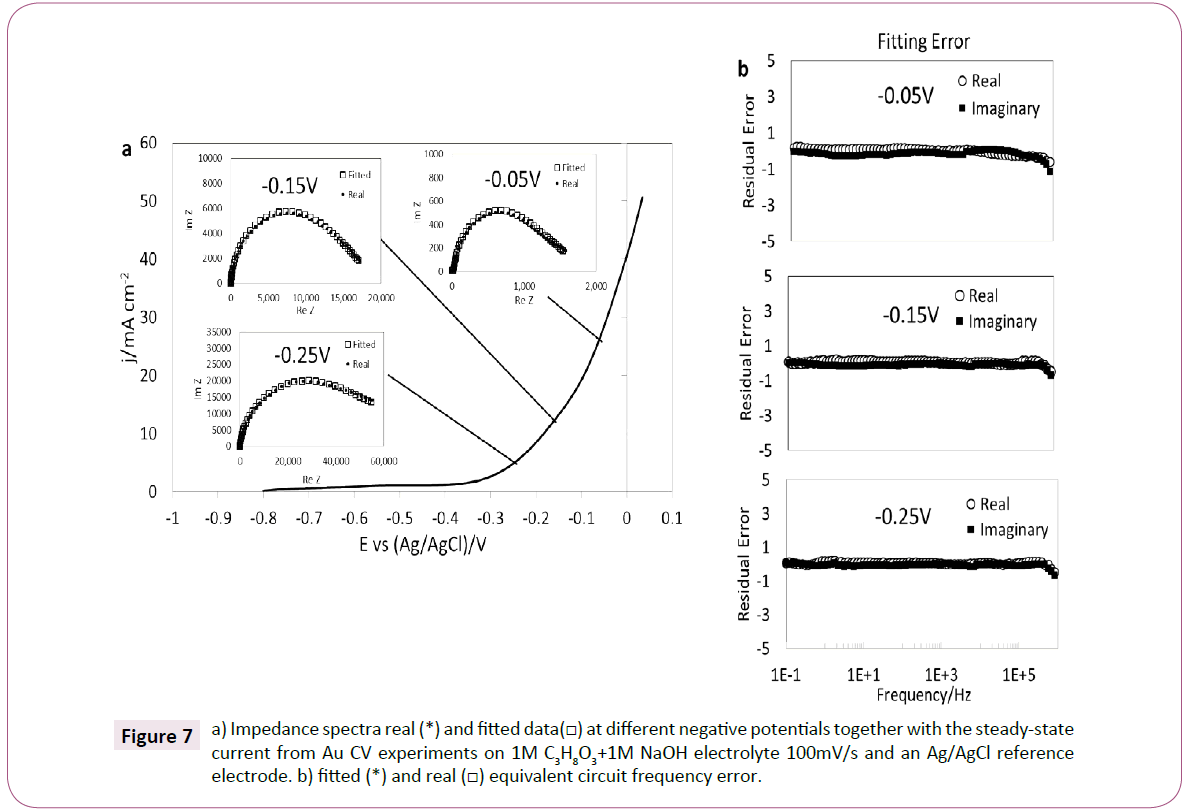
Figure 7: a) Impedance spectra real (*) and fitted data(?) at different negative potentials together with the steady-state current from Au CV experiments on 1M C3H8O3+1M NaOH electrolyte 100mV/s and an Ag/AgCl reference electrode. b) fitted (*) and real (?) equivalent circuit frequency error.
In order to verify the reliability of the data, the relative residuals are plotted in Figure 7b. The data were obtained by Equation (1):
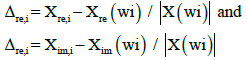 (1)
(1)
Here, Xre,i and Xim,i are the real and imaginary parts of the impedance at a data point (i), Xre(wi), Xim(wi) are the real and imaginary parts of the modeling function for wi. The |X(wi)| is the vector length expressed in the absolute value of the modeling function (X can also represent the statistical behavior of the correlation for fitted data in the circuit). The residuals show a trend that is closer to values between 0-1% for the error in the data fitting, showing that data in the fitting correlation had a low error percentage in all the applied potentials.
Table 1 shows the values of the elements collected from the fitted circuit (resistors, capacitors, and Q element), with an increase of the potential the fitted elements change proportionally. The first resistance (R) is the largest resistance due to the distance of the particles that need to travel to the electrode double layer. The first capacitor (C) represents the double layer capacitance, which is approximately the same at all the potentials, together with the resistance in parallel (R1) that is connected to the second set of resistances-capacitor that are in series(R2C1 and R3C2). In all the cases Q has an n value of closely to 0.5 indicating the presence of the W element.
| V (V) | R (?) | C (F) | R1(?) | R2(?) | C1 | R3(?) | Q (F) | n | C3 (F) | X2 |
|---|---|---|---|---|---|---|---|---|---|---|
| -0.05 | 3.81E+00 | 6.51E-08 | 1.66E+01 | 4.08E+02 | 3.23E-04 | 1.36E+03 | 5.65E-05 | 4.34E-01 | 2.09E-06 | 4.83E-04 |
| -0.15 | 3.68E+00 | 7.36E-08 | 1.69E+01 | 1.20E+02 | 2.22E-04 | 1.95E+04 | 1.08E-05 | 4.02E-01 | 1.40E-06 | 1.45E-04 |
| -0.25 | 3.54E+00 | 7.97E-08 | 1.61E+01 | 2.14E+04 | 1.62E-04 | 6.05E+04 | 4.74E-06 | 4.78E-01 | 1.31E-06 | 1.17E-04 |
Table 1 Values obtained for the circuit fitted for the Impedance spectra of 1 M C3H8O3+1 M NaOH.
Data reliability
In order to validate the circuit modeled. The circuit fitted may be tested using the K-K transform by the method described by Boukamp [29]. Figure 8 shows that the data was transformed according to K-K theory thus confirming that the data is reliable. The capacitors and the resistors presented in this studied are based on fitting and their actual presence in the reaction are not ascertained. However, based on the low-frequency spectra it is possible to observe the presence of capacitors. This could imply that the processes are slow. Also, the analysis of the residual error supports the theory of the presence of the capacitive structures in the reaction. Furthermore, in order to validate the data further Simulink-Matlab was used and the Simulink circuit fitting is shown in Figure 9. The fitted circuit is connected in series with regular AC circuit elements available in the Simulink package, the logic of the system is given by the scope unit. The signal from the circuit indicates that the circuit has a logical frequency output, and it may be used as an electronic system.
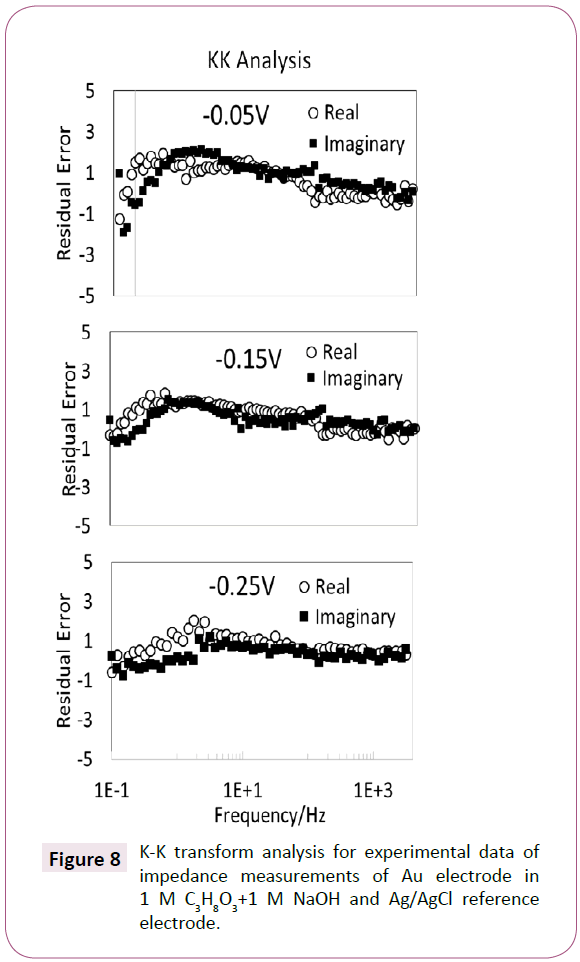
Figure 8: K-K transform analysis for experimental data of impedance measurements of Au electrode in 1 M C3H8O3+1 M NaOH and Ag/AgCl reference electrode.
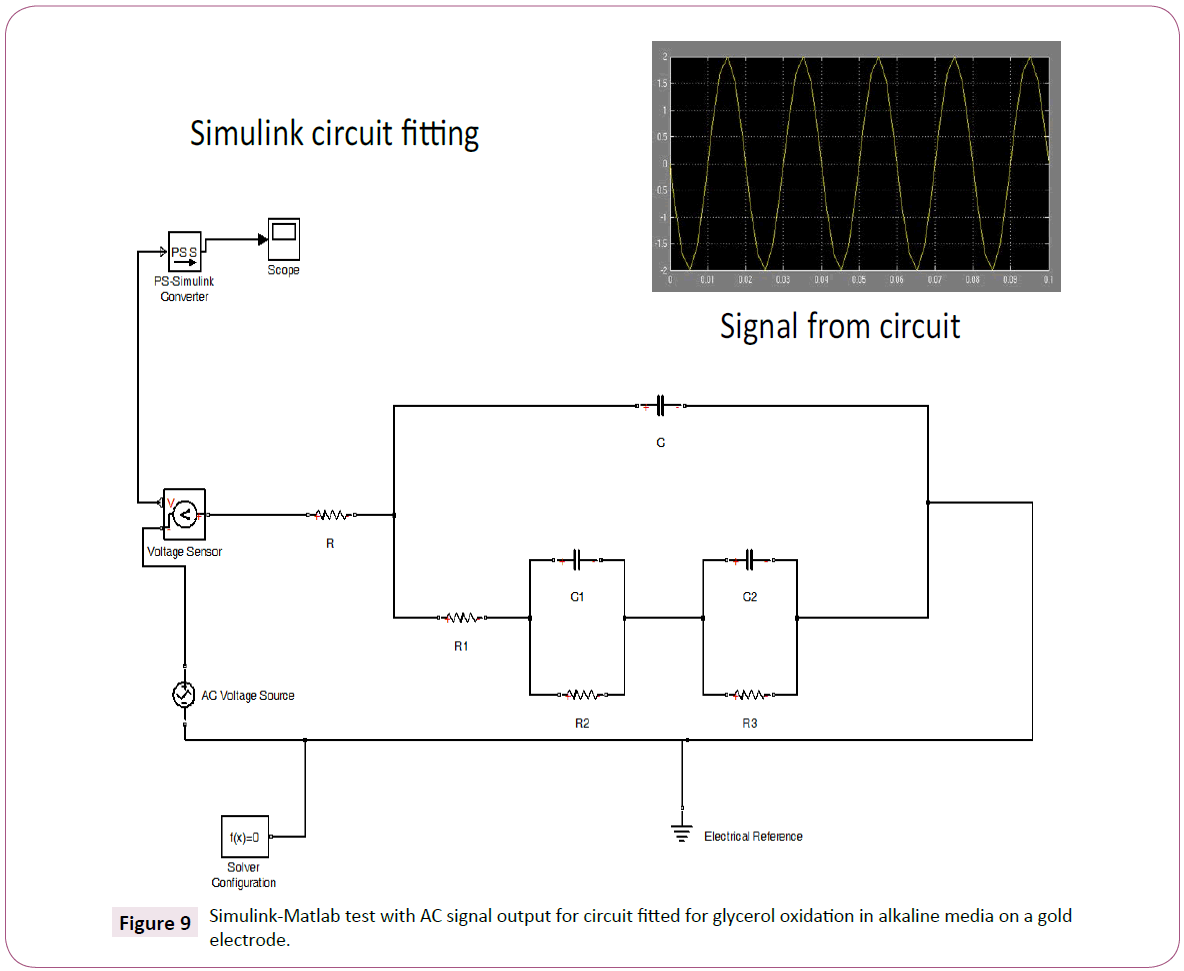
Figure 9: Simulink-Matlab test with AC signal output for circuit fitted for glycerol oxidation in alkaline media on a gold electrode.
Gold coated metals proof of concept
Although the analysis with the gold electrode showed good results for glycerol oxidation in alkaline media, current densities and potentials are still low for a realistic scenario in a fuel cell construction. In order to offer an alternative for the gold electrode, this section presents first, a gold coating method for glassy carbon and the voltammetry test which is compared with gold in alkaline and in acid media to observe the changes in current density and potential in 1 M glycerol aqueous solution. Second, two gold coated metals are tested (Zn-Au and a Cu-Au) in order to compare their electrochemical behavior against pure gold. Zn or Cu may be subject to corrosion but no corrosion was observed in the current experiments.
Gold Coating effect: Glassy carbon (GC) was used as material to deposit gold with the method described in Experimentak Methods, the GC electrode without gold is shown in Figure 10a. A clear layer of gold was deposited (Figure 10b).The gold deposition corresponds to the classical electrochemical-nucleation phenomenon in which the gold ions are traveling through the electrolyte in a diffusion control behaviour [36]. After the electrode was prepared, two cyclic voltammograms were measured one with glycerol in acidic media (Figure 10c) and one with glycerol in alkaline media (Figure 10d). Both voltammograms were compared with the gold electrode.
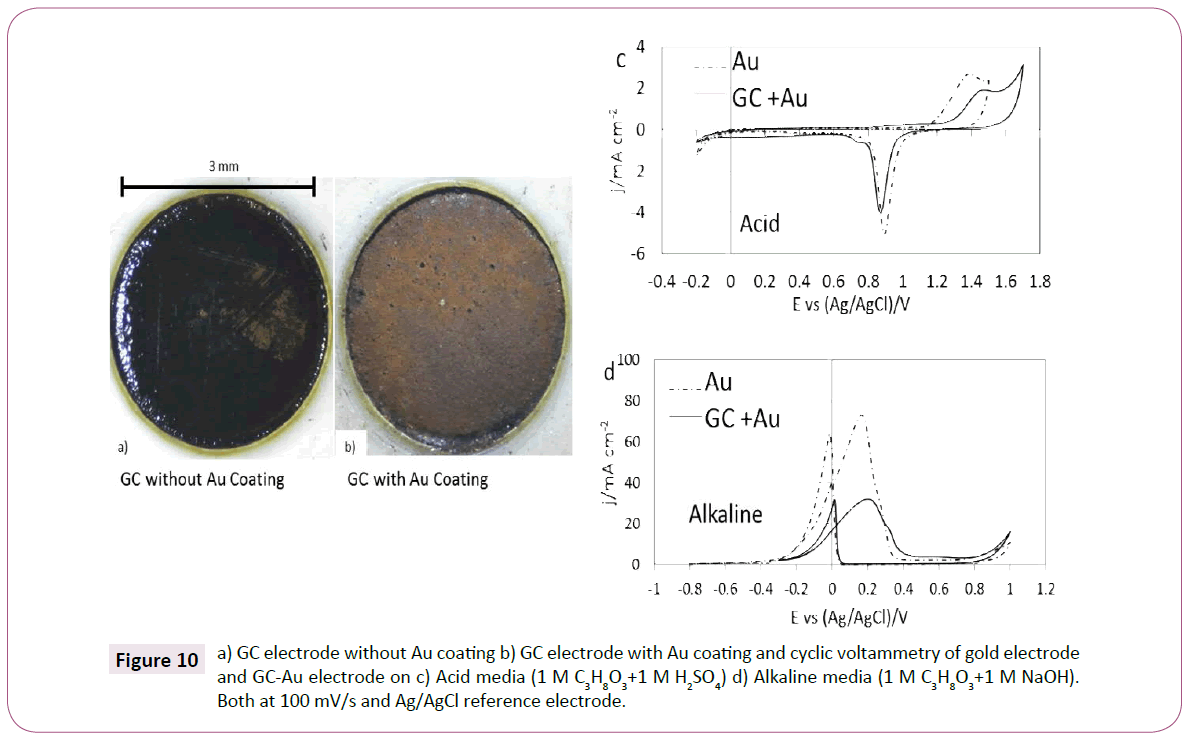
Figure 10: a) GC electrode without Au coating b) GC electrode with Au coating and cyclic voltammetry of gold electrode and GC-Au electrode on c) Acid media (1 M C3H8O3+1 M H2SO4) d) Alkaline media (1 M C3H8O3+1 M NaOH). Both at 100 mV/s and Ag/AgCl reference electrode.
The CV of GC-Au electrode compared with the gold electrode in acid media is shown in Figure 10c. When the potential increases in the positive direction of the scan a first current density peak was observed, possibly, corresponding to gold oxidation, then the potential changed and the gold reduction current density peak was observed. This behavior is typical for a gold electrode in acid media [17]. In both the GC-Au and the gold electrode, cathodic and anodic current density peaks were observed, this is an indication that gold was present on the GC electrode showing similar behavior than the gold electrode. However, it was not observed a clear evidence of glycerol oxidation during the experiment. Also, the GC-Au showed lower current densities than the gold electrode, the reasons for this are may be related to the size of the gold layer, the GC-Au stability and possibly intrinsic resistances between the materials [37].
Figure 10d shows the CV of gold and GC-Au electrodes in 1 M glycerol in 1 M NaOH. It was observed that the GC-Au electrode had a similar behavior than the gold electrode showing two current density peaks, (one for the gold reduction at approximately 0 V and one for glycerol oxidation at approximately 0.2 V vs. Ag/ AgCl). The comparison showed that similar voltammograms may be achieved by using the gold coating method however, lower current densities were observed when using GC electrode as a substrate for gold deposition in comparison with the gold electrode.
In both acid and alkaline media, the CV behavior of gold electrode was observed when using GC-Au electrode. Furthermore, it is suggested that using GC as a substrate material for gold plating has an effect on the current density, potential, and the capacitive plateau during voltammetry. This effect may be due to the electrochemical nature of GC, which may affect the normal behavior of the gold electrode for glycerol oxidation [18], similar observations were done by Othman [38], in which Au-PVC electrode was used for glycerol oxidation and it was observed that the gold coated material can mimic the electrochemical behavior of gold.
The Zn-Au electrode
Based on the observations done with the GC electrode. It is plausible that changing the substrate material for gold electrodeposition may affect the current density and potential in the voltammetry of glycerol in alkaline media. A Zn-Au electrode was prepared using the same procedure for the GC-Au electrode. The electrode was checked visually before and after been in the aqueous solution of AuBr2 and it was observed a change in color on the surface of the zinc electrode, the change may be related to zinc oxidation [39] and also, to a displacement of zinc ions for gold ions [40]. In order to observe if gold is presented on the surface of the zinc electrode voltammetry was used. Furthermore, the effect of cyclic voltammetry cycling is presented in Figure 11. Thirty cycles are shown at a scan rate of 100 mV/s, the shape of the voltammetry is similar to the gold electrode implying, that possibly a gold layer was created on the surface of the zinc electrode and that glycerol oxidation may be possibly achieved similarly than with gold. After each cycle in the voltammetry, the current density increased, having a similar effect than the GC-Au electrode. This observation also suggests that the Zn- Au electrode has a layer of gold deep enough to continue the glycerol oxidation during each cycle.
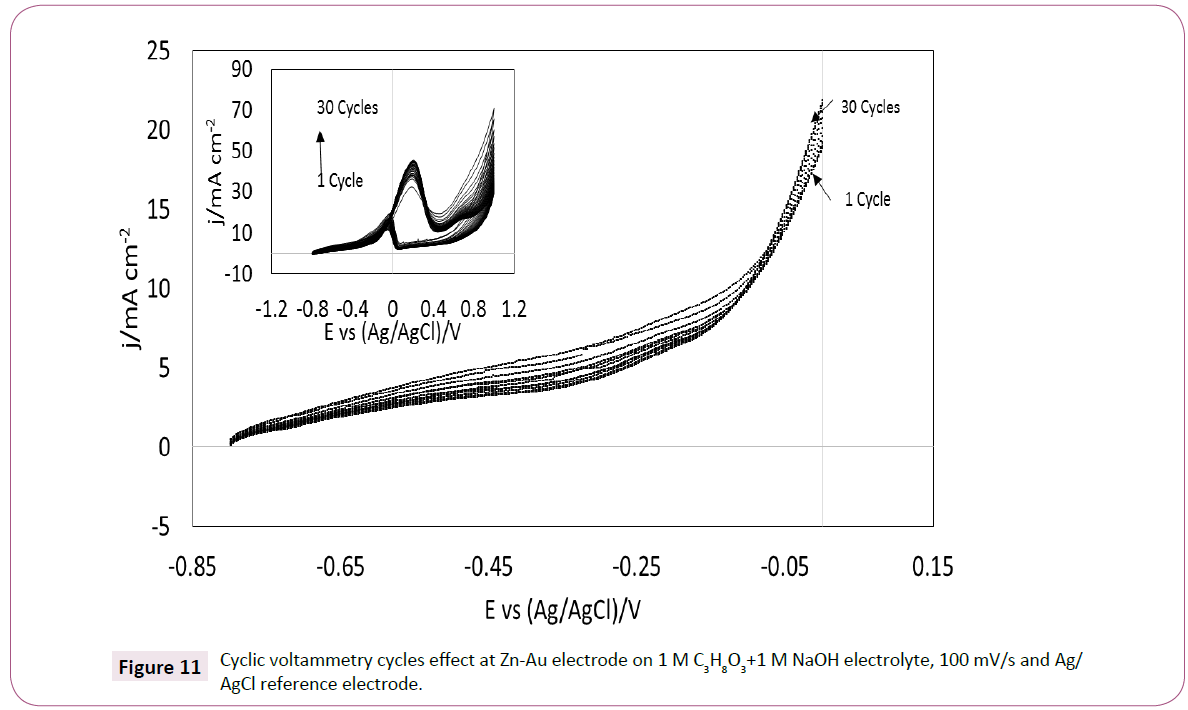
Figure 11: Cyclic voltammetry cycles effect at Zn-Au electrode on 1 M C3H8O3+1 M NaOH electrolyte, 100 mV/s and Ag/AgCl reference electrode.
To understand more about the kinetics of the glycerol oxidation in alkaline media at Zn-Au electrode, the influence of scan rate is studied in Figure 12a. Similar to the GC-Au electrode, the current density gradually increased with the scan rates, and the correlation between the scan rate and the current density at -0.15 V is linear at a scan rate coefficient of 1/5 (I vs. v1/5) and not at 1/2 (Figure 12b). Other scan rates exponential coefficients were tested but not linearity was observed. This observation may suggest that the glycerol oxidation using the Zn-Au electrode is not totally controlled by diffusion, as in the case with the GC Au electrode. Also, with the increase of scan rate the potential is negatively shifted. The varied potential may represent both an alteration in the Zn-Au surface due to the interaction with glycerol and OH- ions [34], and the interaction between gold and zinc that shifted the potential to negative values, this possibly related to the negative electrochemical potential of zinc and possible side reaction of zinc in alkaline media forming zinc oxide and zinc hydroxide both with larger negative potential than zinc as shown in Equation 2. However, the actual presence of these reactions was not characterized in detail in this study.
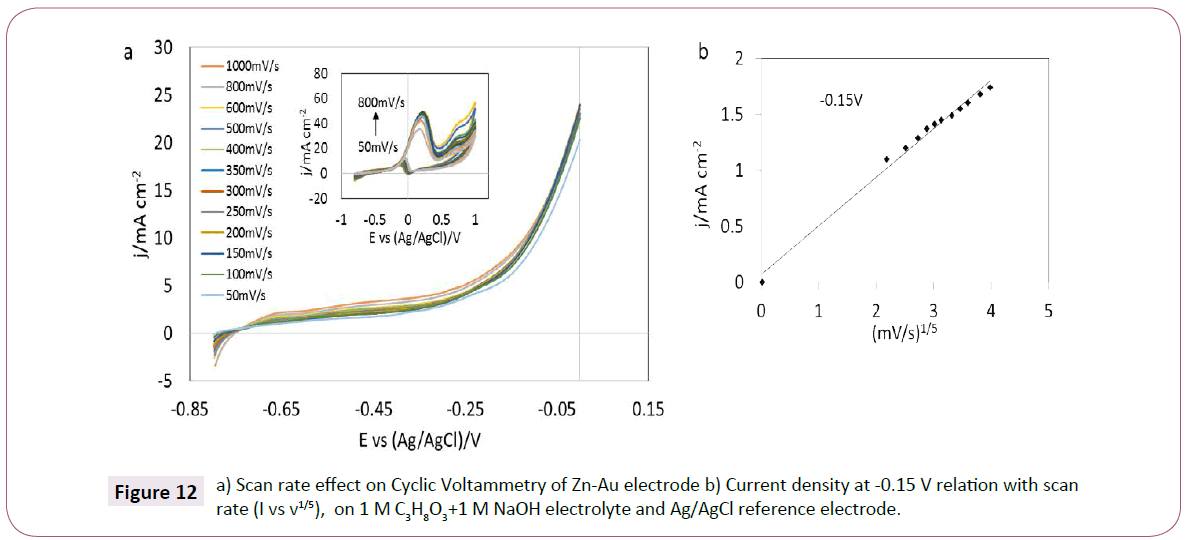
Figure 12: a) Scan rate effect on Cyclic Voltammetry of Zn-Au electrode b) Current density at -0.15 V relation with scan rate (I vs v1/5), on 1 M C3H8O3+1 M NaOH electrolyte and Ag/AgCl reference electrode.
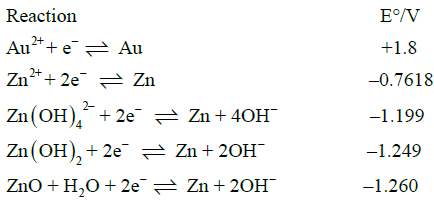 (2)
(2)
The Cu-Au electrode
The Cu-Au electrode was studied using the same conditions for the GC-Au electrode and the Zn-Au electrode. The electrode was checked visually before and after being in the aqueous solution of AuBr2 the color on the surface of the copper electrode changed. The change may be possibly related to copper oxidation [41]. Also, it may result from displacement of copper ions for the more noble gold ions [40]. The effect of cyclic voltammetry cycling is presented in Figure 13. The shape of the voltammetry is similar to the gold electrode the GC-Au and Zn-Au electrode, showing that a gold layer was created on the surface of the copper and that similar shape can be observed in the voltammetry of glycerol oxidation as compared with the Au electrode. After each cycle in the voltammetry, the current density increased similarly to the GC-Au and the Zn-Au electrodes. This observation also suggests that the Cu-Au electrode had enough gold to continue the constant cycling.
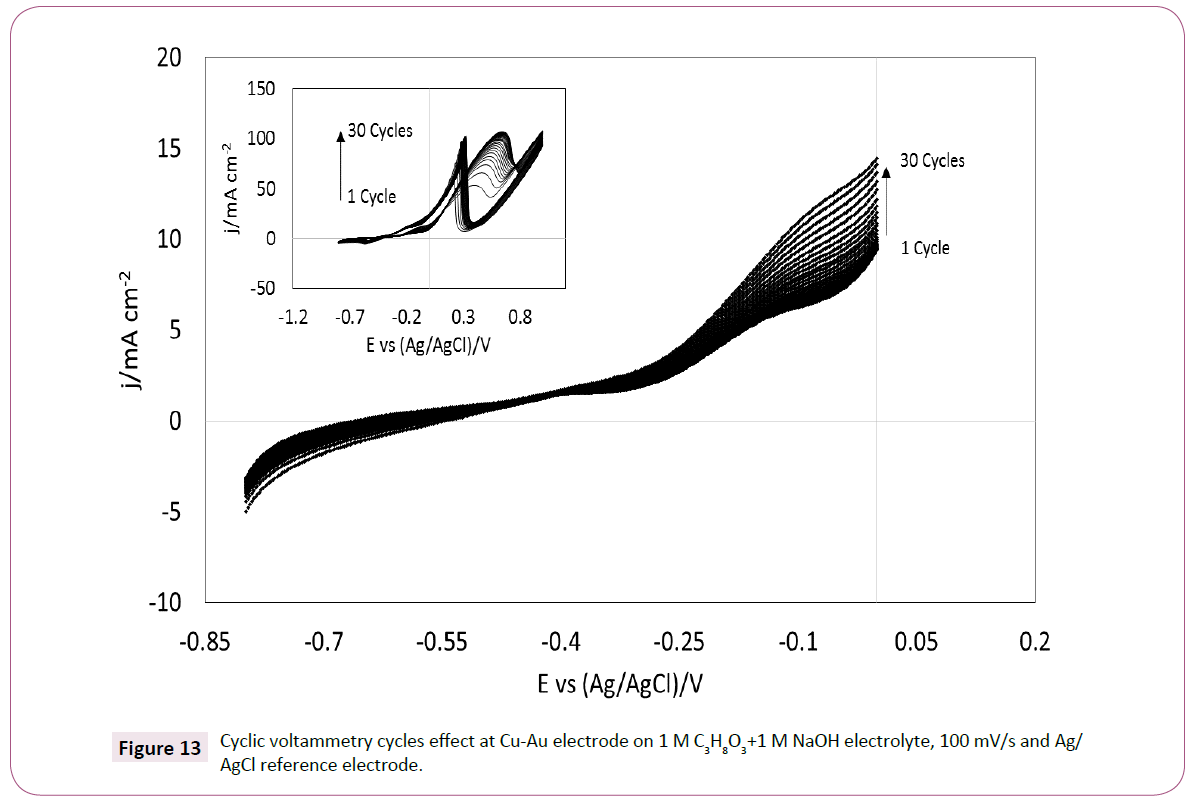
Figure 13: Cyclic voltammetry cycles effect at Cu-Au electrode on 1 M C3H8O3+1 M NaOH electrolyte, 100 mV/s and Ag/AgCl reference electrode.
The influence of scan rate is shown in Figure 14a. It can be seen that the current density gradually increased with the scan rates. The correlation between the scan rate and the current density was measured at a potential of -0.15 V vs. Ag/AgCl and it was found to be linear at a scan rate coefficient of 1/5 (I vs. v1/5) and not at 1/2 (Figure 14b). These observations suggest that the glycerol oxidation at Cu-Au electrode is not totally controlled by diffusion. Also, with the increase of scan rate the potential is negatively shifted similarly to the Zn-Au electrode. The varied potential possibly represents both an alteration in the coppergold surface due to the interaction with glycerol and OH- ions produce during the voltammetry [34]. The voltammetry of the Cu-Au shows a change in the voltammetry shape towards more negative potential. This may be explained by the electrochemical potential of copper and other copper ions in alkaline solution (copper oxide and copper hydroxide) which are more negative than gold in alkaline solution (Equation 3).
Figure 14: a) Scan rate effect on Cyclic Voltammetry of Cu-Au electrode b) Current density at -0.15 V in relation with scan rate ( I vs. v1/5), on 1 M C3H8O3+1 M NaOH electrolyte and Ag-AgCl reference electrode.
 (3)
(3)
Comparison of Au, Zn-Au and Cu-Au electrode
The behavior of Au, Zn-Au, and Cu-Au electrodes is compared in Figure 15a, the comparison has been done using the ideal plot for an anode material in a glycerol fuel cell (Figure 1), using the region between -0.8V and 0 in the cyclic voltammetry for glycerol oxidation in alkaline aqueous solution. Both Zn-Au and Cu-Au have similar behavior than the Au electrode in a solution of 1 M glycerol and 1 M NaOH at 100 mV/s and Ag/AgCl as a reference electrode. When analyzing the current densities at a potential of -0.15 V (Figure 15b), the largest current density observed is from the Zn-Au electrode with a value of 7 mA cm-2.The lowest current density observed is from the Au electrode with 0.7 mA cm-2 at a potential of -0.45 V. For all the electrodes studied the current density decreases while the potential goes to negative values. This behavior is lower in the Zn-Au electrode and the Cu-Au electrode when compare with the Au electrode. The experiment showed that changing the metal for gold deposition may have an effect on current density and potential and that Zn-Au and Cu-Au electrodes are possible materials to be used for glycerol oxidation in alkaline media.
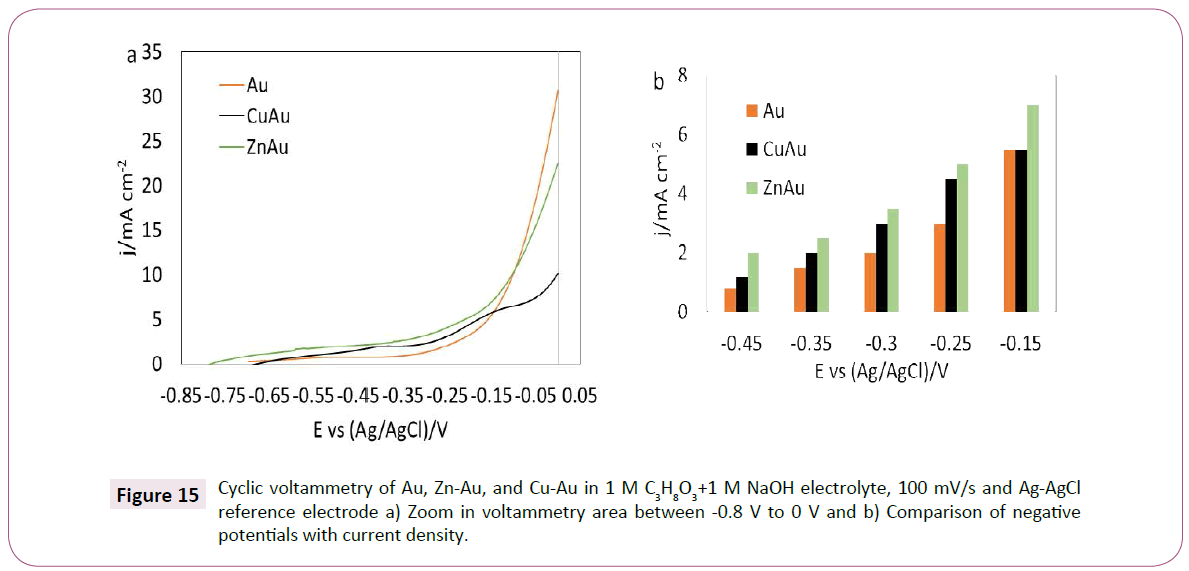
Figure 15: Cyclic voltammetry of Au, Zn-Au, and Cu-Au in 1 M C3H8O3+1 M NaOH electrolyte, 100 mV/s and Ag-AgCl reference electrode a) Zoom in voltammetry area between -0.8 V to 0 V and b) Comparison of negative potentials with current density.
Table 2 shows a list of different catalyst for glycerol oxidation compared with the catalyst used in our research. The experimental conditions show that different electrolyte compositions are used for the study of the glycerol oxidation. Furthermore, the comparison of the performance of the catalyst has not been studied in detail to our knowledge. E.g. the Pt, and Pd catalyst have different alkaline media (NaOH or KOH), temperature and pH. Nevertheless, in the analysis of different authors suggest that the glycerol oxidation is a complex reaction that is influenced by physical, chemical and electrochemical conditions and finding the right set of characteristics is still a challenge to research.
| Experimental conditions | |||||
|---|---|---|---|---|---|
| Catalyst | Supported material | Temperature Co | pH | Main electrolyte | Observations |
| Pt | MCN (mesoporous carbon nitrides) 9 | 60 | Neutral | 0.3M Glycerol | There is a decrease of catalytic activity after reusing of the catalyst also the experiments were performed in a base free environment. |
| Pt-Ru | MWCNT (multi-walled carbon nanotubes) 10 | 20-90 | Alkaline | 0.7M Glycerol+2M KOH | Superior catalytic activity than Pd -MWCNT |
| MWCNT (multi-walled carbon nanotubes) 10 | 20-90 | Alkaline | 0.7M Glycerol+2M KOH | It is observed higher current densities at temperatures of 80 Co | |
| Pd | CCE (Carbon ceramic electrode) 11 | 25 | Alkaline | 0.5 m Glycerol+0.3 m NaOH | The concentration of NaOH influences the current density of glycerol oxidation |
| Au | Carbon17 | 50-60 | Alkaline | 0.1M Glycerol+0.1 NaOH+0.1M Tartronic Acid | The added tartronate improved the glycerol oxidation due to selectivity of Au for tartronate ions at high temperature |
| MnO2-Carbon 22 | 60 | Alkaline | 0.5M Glycerol+1M KOH | MnO2 gave support for gold nanoparticles and better electrode stability | |
| This Research | |||||
| Au | Au GC Zn Cu |
23 | Alkaline | 1M Glycerol + 1M NaOH | High catalytic activity Similar catalytic activity than gold Higher current densities are observed and potentially shifted to negative values Higher current densities are observed and potentially shifted to negative values |
Table 2 Comparison of different catalyst for glycerol oxidation from literature with the present study.
Proof of concept: Chronoamperometry discharge analysis
A prototype system was built to observe the discharge performance of the anode electrodes studied in this paper. Copper and zinc foils of 20 cm2 with electrodeposit gold were used as anodes for glycerol oxidation using the process described in Experimental Methods. The cathode was carbon graphite for the oxygen reduction reaction. The graphical representation of the cell is shown in Figure 16a. The two materials were immersed in a solution of 1 M glycerol and 1 M NaOH. Two different potentials were tested for 900 seconds in each cell (-0.05 V and -0.15 V), the values were selected base in the results of EIS and CV analysis, taking into account the lowest resistance observed. The results are shown in Figure 16. Overall, it is observed that when the load potential is increased the current density decreases. In the case of the gold cell (Figure 16 b), at -0.05 V the current density average discharge is equal to 0.07 mA cm-2 and at -0.15 V is equal to 0.01 mA cm-2. In the case of the Zn-Au electrode (Figure 16c), the current density changes from 1.5 mA cm-2 (at -0.05 V) to 1 mA cm-2 (at -0.15 V) and for the Cu-Au electrode (Figure 16d) the current density average discharge is 3 mA cm-2 (at -0.05 V) and 1.5 mA cm-2 (at -0.15 V).
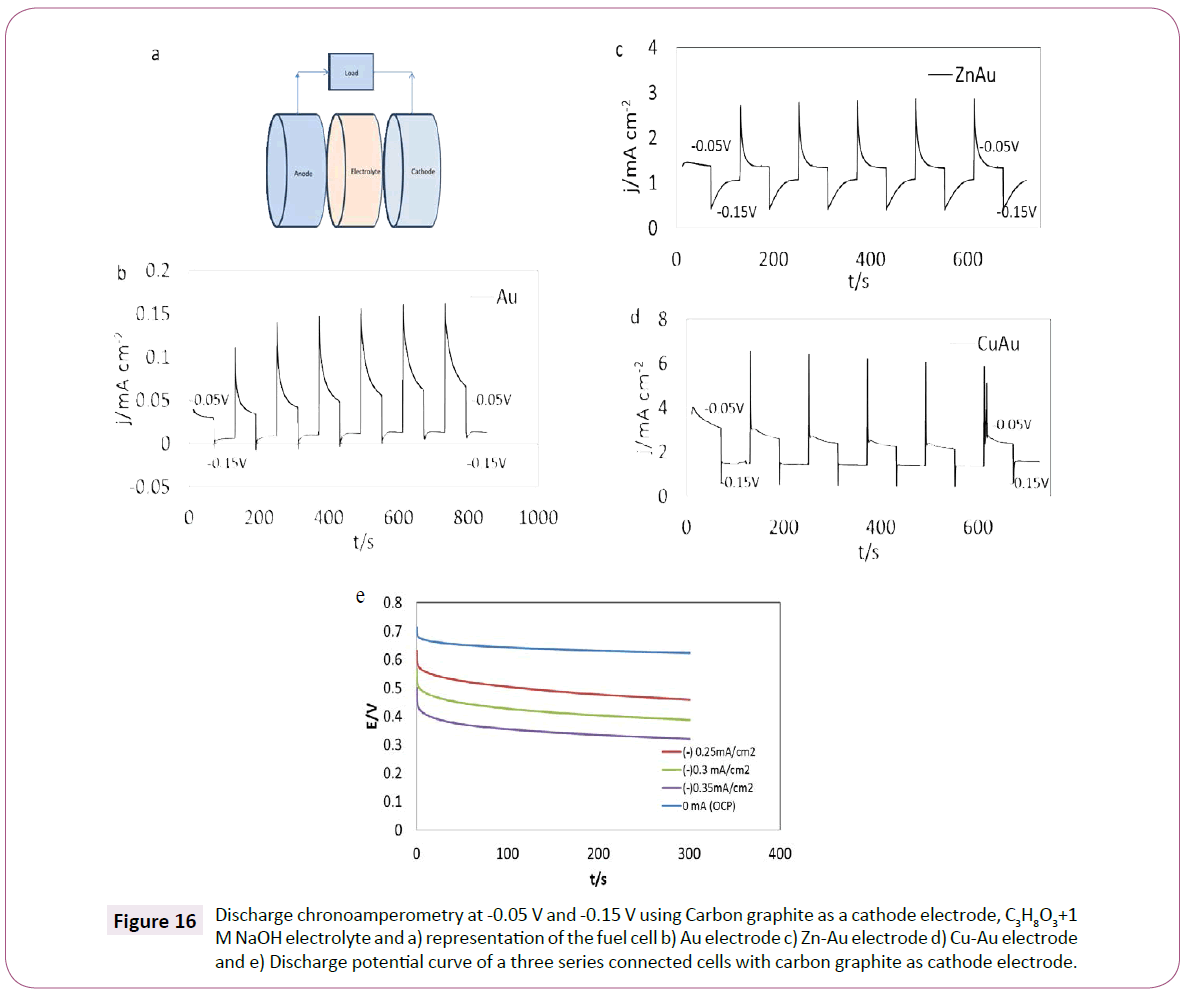
Figure 16: Discharge chronoamperometry at -0.05 V and -0.15 V using Carbon graphite as a cathode electrode, C3H8O3+1 M NaOH electrolyte and a) representation of the fuel cell b) Au electrode c) Zn-Au electrode ) Cu-Au electrode and e) Discharge potential curve of a three series connected cells with carbon graphite as cathode electrode.
The difference between the cells current densities is possibly related to resistance that can be created between the electrodes. In the three cells tested gold was observed to have the lowest current output at the given potentials, followed by Zn-Au and the highest current density was observed in the Cu-Au electrode. However, in the case of Cu-Au at the end of the last discharge, it is observed a double line, indicating conductivity problems with the cell, this could be due to surface changes in the electrode after the discharge and possible surface oxidation (Cu2O and/or Cu(OH)2). Further experimentation needs to be done in order to know the exact maximum capacity of the electrode and the gold bonding. Figure 16e shows the analysis of a three electrode fuel cell connected in series, the test was performed using different current densities in order to observe the discharge potential response and the stability of the cathode electrode for a period of 300 s. While the current density increases the potential of the cell reduces constantly. At 0 mA cm-2, the voltage of the cell is stable at 0.7 V. However, the potential changes during the time when a load is not applied. This behavior suggests that the stability of the carbon electrode is changing during the 300 s experiment.
Conclusions
Our analysis shows that gold has the highest current density for glycerol oxidation in alkaline media when compared with another catalyst at a potential of -0.2 V (Pt, Ag, GC and Cu). It is further observed that gold surface can change in cyclic voltammetry. The current density shows linear behavior with the scan rate at an exponential value of 1/5 and not with the square root. One equivalent circuit fitted the data well at potentials of -0.05 V,- 0.15 V and -0.25 V vs. Ag/AgCl, with resistors and a Warburg element in parallel with the double layer capacitance. Elements are related to the possible presence of hydroxypyrovate and oxalate ions, our results were consistent with the low-frequency error fitting analysis (10-4), AC Simulink-Matlab fitting responds and the Kronig-Kramers transform test. The Zn-Au and the Cu- Au electrodes showed voltammetry behavior similar to the gold electrode in cyclic voltammetry at the different scan rate. The discharge chronoamperometry test showed that the Zn-Au and Cu-Au electrode had higher current densities than the gold electrode at a potential of -0.25 V vs. Ag/AgCl (5 mA cm-2, 4.5 mA 18 cm-2, and 3 mA cm-2 respectively). Au, Zn-Au, and Cu-Au are possible candidates for glycerol oxidation technologies and the gold coated metals are possibly suitable electrodes for building a fuel cell. In this study, the air electrodes used in the fuel cell test were limited instability and in our next study, we will address stable air electrodes for our glycerol fuel cell approach.
Upon success in prototyping, we aim to possibly use fuel cells as backup systems in microgrids or as an alternative to replace diesel generators in some situations. Although, more R&D is needed to set this insight too. In the meanwhile, our group has started simulations with these propose glycerol fuel cell in order to anticipate on this scenarios.
Acknowledgment
This work was sponsored by the STW-I-Care 11854 project and Dr Ten BV, in The Netherlands as part of its ongoing fuel cell development research and demonstration of storage systems for smart grid integration. Also special thanks to MSc. Miheer Vaidya and the Delft University of Technology in The Netherlands.