Research Article - (2023) Volume 9, Issue 2
A Preliminary Study on the Utility of Root® with SedLine® for Sleep Evaluation in Critically Ill Patients: Comparison with Polysomnography and the Richards-Campbell Sleep Questionnaire
Kanae Nagatomo1*,
Masamitsu Sanui1,
Tomoyuki Masuyama2,
Yusuke Iizuka1 and
Junji Shiotsuka1
1Department of Anesthesiology and Critical Care Medicine, Jichi Medical University Saitama Medical Center, Japan
2Department of Emergency and Critical Care Medicine, Jichi Medical University Saitama Medical Center, Japan
*Correspondence:
Kanae Nagatomo,
Department of Anesthesiology and Critical Care Medicine, Jichi Medical University Saitama Medical Center,
Japan,
Email:
Received: 13-Feb-2023, Manuscript No. IPJICC-23-15695;
Editor assigned: 15-Feb-2023, Pre QC No. IPJICC-23-15695 (PQ);
Reviewed: 01-Mar-2023, QC No. IPJICC-23-15695;
Revised: 06-Mar-2023, Manuscript No. IPJICC-23-15695 (R);
Published:
13-Mar-2023, DOI: 10.35248/2471-8505.23.9.012
Abstract
Introduction: To date, there has not been a practical evaluation of patient sleep in critical care units, where sleep disturbance is commonly found. This study aimed to validate a portable electroencephalography monitor, as a sleep monitoring device, against Polysomnography (PSG) for objective sleep evaluation and the Richards-Campbell Sleep Questionnaire (RCSQ) for subjective sleep quality in critically ill patients.
Methods: In this observational study, the sleep pattern of 10 adult patients staying in an ICU for at least 72 hours was evaluated for 24 hours using PSG and the Patient State Index (PSI) from SedLine. Additionally, their nighttime sleep was subjectively scored using the RCSQ to compare with the objective parameters.
Results: Sleep architecture from PSG showed an increase in light sleep and a prominent decrease in restorative sleep, despite preserved quantity of nocturnal sleep. Based on PSG results, PSI was distributed in accordance with sleep depth. Subjective quality of nocturnal sleep from the RCSQ was correlated with the PSI (r=-0.816, 95% Confidence Interval [CI]: -0.955 to -0.383), as well as with the nighttime stage N2 ratio from PSG alone (r=0.741, 95% CI: 0.209 to 0.935) or combined with restorative sleep (r=0.801, 95% CI: 0.347 to 0.951). The cutoff value of PSI to distinguish between the stage N1 and N2 was 67.0 (Specificity, 0.641; Sensitivity, 0.845 with area under curve [AUC] of 0.818).
Conclusion: SedLine is considered a feasible and valid instrument for sleep quality assessment in ICU patients.
Keywords
ICU; Sleep evaluation; Portable electroencephalography monitor (SedLine); Polysomnography; Richards-Campbell sleep questionnaire
Abbreviations
(AASM) American Academy of Sleep Medicine; (ARI) Arousal Index; (AUC) Area under the Curve; (BIS) Bispectral Index; (EEG) Electroencephalography; (EMG) Electromyography; (EOG) Electrooculography; (ICU) Intensive Care Unit; (IQR) Interquartile Range; (MV) Mechanical Ventilation; (NREM) No-Rapid Eye Movement; (PSG) Polysomnography; (PSI) Patient State Index; (RASS) Richmond Agitation-Sedation Scale; (RCSQ) Richards-Campbell Sleep Questionnaire; (REM) Rapid Eye Movement; (ROC) Receiver Operating Characteristic; (SE) Sleep Efficiency; (TST) Total Sleep Time; (WK) Wakefulness
Introduction
Sleep disturbance, with its adverse implications, has been commonly reported in critically ill patients [1-7]. Sleep disturbance can exacerbate disease, such as by immune suppression, catabolic unbalance, respiratory distress, or cardiovascular activation [1-6]. It can also cause deterioration of cognitive function, including delirium, which may distress patients after their discharge from Intensive Care Units (ICU), a clinical scenario known as post-intensive care syndrome [2-6,8,9]. Polysomnography (PSG) is the gold standard for objective sleep evaluation. It can be used to identify changes in sleep architecture such as severe fragmentation from frequent arousals and awakenings, an increase of light sleep and a decrease of restorative sleep as well as excessive daytime sleeping despite normal night sleep quantity [1-7]. These types of changes can cause sleep disturbance in critically ill patients. However, PSG is impractical for routine real-time sleep assessment due to the complexity of its interpretation, its technical difficulty, high cost, and intolerance by some patients [3-10]. In contrast, subjective reporting tools, such as the Richards-Campbell Sleep Questionnaire (RCSQ), have also identified poor sleep quality in ICU patients [1,11-13].
Although a correlation between the RCSQ and PSG metrics has been established, self-assessment can be difficult without patient cooperation, especially in cases of cognitive impairment or when sedatives are required [5,10,12-14]. Root® with SedLine® (SedLine; Masimo Corp., Irvine, CA, USA) is widely used as a brain function monitor during anesthesia. The platform uses a SedLine Sensor with 4 bilateral frontal Electroencephalography (EEG) channels and 2 Electromyography (EMG) channels in a single sticker that is attached to the forehead. This 6-electrode set placed as recommended by the American Academy of Sleep Medicine (AASM) for sleep monitoring, captures a symmetrical bilateral frontal array of raw EEG and EMG signals [15]. These signals are subsequently converted into a Patient State Index (PSI), ranging from 0 to 100. The PSI values are indicative of the depth of general anesthesia or sedation, similar to the Bispectral Index (BIS) that also provides information regarding the depth of general anesthesia or sedation, scoring a symmetrical bilateral frontal array of raw EEG and EMG signals from 0 to 100. Sleep EEG monitoring using SedLine has already been validated against PSG, but the feasibility of its clinical use remains to be established [16]. To the best of our knowledge, there have been no previous reports investigating the practical utility of this instrument for evaluating the quality of sleep in critically ill patients. The quality of sleep in this population can be influenced by a myriad of clinical complications; therefore, there is an urgent need to establish novel techniques for measuring their sleep quality. In this study, we evaluated sleep in critically ill patients using SedLine and compared the results with 24 hour PSG and subjective sleep reports during nighttime using the RCSQ. We aimed to assess the feasibility and validity of the PSI in a critical care setting, where the conduction of continuous PSG or self-sleep assessment may be challenging. We hypothesized that the PSI would provide comparable results to both, the PSG sleep stages and subjective measures of sleep quality as assessed by the RCSQ. The PSI was also hypothesized to be a useful measure to identify PSG sleep stages.
Materials and Methods
Study Setting and Sample
This study was conducted in the general ICU at Jichi Medical University Saitama Medical Center, a 600 bed tertiary teaching hospital in Saitama, Japan, from March 2017 to October 2017. The study was conducted in an 8 bed ICU area, where patients have the most complicated care conditions. In this ICU, exclusive intensive care doctors treat perioperative patients of the cardiovascular or other surgical departments with severe complications as well as internal patients with acute respiratory failure, septic shock, and other critical diseases requiring mechanical ventilation, continuous renal replacement therapy, or extracorporeal devices. The registered nurse-to-patient ratio was 1:1 or 1:2 depending on their shifts or patients’ conditions. All patients were 20 years or older and stayed in the ICU for at least 72 hours. These patients were evaluated using both 24 hour sleep SedLine and PSG, and the RCSQ to evaluate nighttime sleep (21:00 hours to 05:59 hours) quality. Patients with cognitive impairment, psychiatric disorders, dementia, alcohol or drug abuse, post-cardiopulmonary arrest, obvious delirium on ICU admission, and those who were unable to communicate in Japanese were excluded. This study received approval from the Institutional Review Board of Jichi Medical University Saitama Medical Center (S17-134), and informed written consent was obtained from all the patients or from their family representatives. This study was part of an interventional trial registered with the University Hospital Medical Information Network Individual Clinical Trials Registry (UMIN-CTR000026350, http:// www.umin.ac.jp/icdr/index-j.html) on March 1, 2017.
Data Collection
Patients were monitored for one 24 hour period with a SedLine sensor. Simultaneous PSG (Alice6 LDx; Philips Respironics, Murrysville, PA, USA) was acquired. EEG electrodes (F4/M1, C4/ M1, O2/M1, F3/M2, C3/M2, O1/M2) were placed by trained technicians according to the International 10 to 20 System and the EMG electrodes were positioned on the right, left, and central chin. Electrooculography (EOG) was used for monitoring right and left eye movements and electrocardiography (ECG; LeadⅡ) for monitoring cardiac physiology. The recording began between 09:00 and 18:00. SedLine provides recording of the PSI in addition to frontal EEG data. The EEG data acquired with the SedLine were not analyzed. We excluded all subjects where data from either the PSI or the PSG were insufficient (less than several hours of the 24 hour assessment period or lacking multiple hours of nighttime data). Patients were asked to fill out the RCSQ to assess their nighttime sleep before or after completion of the PSG. RCSQ assesses sleep depth, sleep latency, frequency of awakenings and latency after awakenings, and sleep quality using a 100 mm visual analog scale. Higher scores indicate satisfactory sleep. The total RCSQ score is calculated as the average of the ratings on each of these 5 questions. The original English version of the RCSQ was translated into Japanese and previously validated [17]. We provided the Japanese questionnaire with large letters and illustrations in order to make it easier for critical care patients to see and answer by pointing with their finger, assisted by a nurse if necessary. Demographic and clinical data were collected from the patients’ records, and the APACHE II score of illness severity on admission was calculated as well.
Data Analysis
PSI values were continuously recorded every 2 seconds during the monitoring period and stored in the SedLine hard disk. The data were exported after every 24 hour recording period through dedicated PC software (Masimo Instrument Configuration Tool). Concomitant 24 hour PSI data, measured at the same time as PSG, were extracted to be validated against PSG results.
The nighttime SedLine data was used for validation against the RCSQ. PSG recordings were checked manually in 30 seconds epochs using the AASM scoring manual version 2.4 by an experienced Registered Polysomnographic Technologist® from an external expert agency who had analyzed a total of 4500 cases at that time [15]. The technologist received anonymous data in order to protect patients’ privacy. Summary information, such as disease, critical status, ventilation status, and medication, was provided before each evaluation. For each epoch, PSG recordings were scored for Wakefulness (WK) and stages N1, N2, and N3 of non-REM (NREM) sleep, and REM sleep. Total Sleep Time (TST) was calculated from the total number of NREM and REM epochs, and Sleep Efficiency (SE) was calculated from the TST divided by 1440 for 24 hour data and by 540 for nighttime data. Arousal index, defined as the number of arousals per hour, and the frequency of transitions to another sleep stage during night time were also determined. Sleep parameters, such as sleep latency or wakes after sleep onset were not calculated, as sleep cycles were often seen before lights-out (21:00) and after lights-on (06:00). To compare PSI and PSG sleep stage information, 30 seconds epochs of PSG data were transformed into 15 windows of 2 seconds data. For example, if 30 seconds epoch was classified as stage N2, all 15 (2 seconds) windows of this 30 seconds epoch were labeled as stage N2, which is methodologically similar to a previous study validating the BIS and PSG staging [18]. When PSI data were not obtained due to mechanical or sweating artifacts, both PSI and PSG data for those epochs were excluded from the analysis. Pearson’s rank correlation coefficient, derived from the average of nighttime PSI and the ratio of nighttime PSG sleep stages, was used to compare RCSQ among the subjects. For statistical analyses, we used EZR (R software version 3.4.1) [19]. Nominal variables are shown in numbers (n) with percentages (%). For numerical variables, we present median values with the 25% to 75% Interquartile Range (IQR). P values<0.05 were considered statistically significant.
Results
Patient Characteristics
Of the 481 patients admitted to the ICU throughout the duration of this study, we report the data of 10 patients (Figure 1). There were 124 patients who met the inclusion criteria; 34 declined PSG monitoring, and 16 were discharged before they could be enrolled. Thus, 74 patients were eligible, but 59 could not be enrolled because of their treatment schedule or unavailability of the technicians or the researchers. As a result, 15 patients were enrolled; however, 1 patient requested removal of the PSG once the recording began, there were 3 patients who had to be removed due to unexpected attachment failure of PSG electrodes, and another patient with insufficient PSI data. Therefore, we report the data from 10 patients (Figure 1).
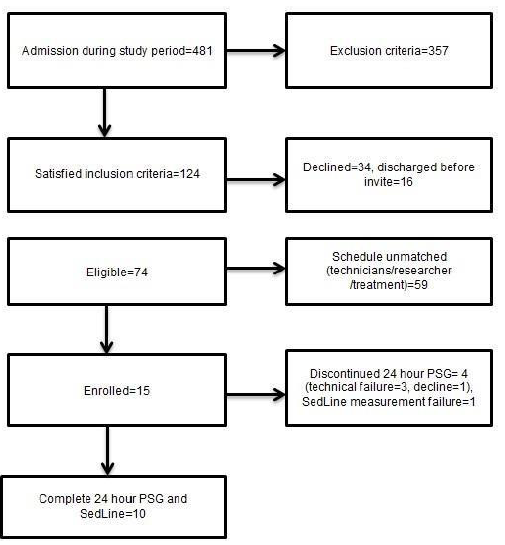
Figure 1: Study flow diagram
The characteristics of the 10 patients in this study are summarized in Table 1. The majority were postoperative patients (90%), with 3 being emergency admissions. Assessment using the Richmond-Agitation Sedation Scale (RASS) indicated alertness or light sedation in all the patients, whereas 3 patients were delirious. A summary of the sleep parameters as assessed by 24 hour PSG, PSI, and by the RCSQ is shown in Table 2. Our PSG assessments revealed an increase in light sleep and a reduction of restorative sleep, despite a preserved quantity of nocturnal sleep. All the 10 participants had certain epochs of WK, N1 and N2, whereas no N3 was found in 4 participants and no REM sleep in 5 others. The distribution of PSI compared to PSG is described (Figure 2) (Tables 1 and 2).
| Total n=10 |
Number or Median |
% or IQR |
| Male, n |
8 |
80.00% |
| Age, year (median) |
72 |
67.3-77.5 |
| APACHE II on admission (median) |
18 |
15.3-23.3 |
| Medical, n |
1 (Septic shock) |
|
| Perioperative, n |
9 |
|
| Cardiovascular, n |
8 |
|
| Hepatic resection, n |
1 |
|
| Emergency, n |
3 (Cardiovascular) |
|
| Length of MV, day (median) |
2 |
1.3-7.8 |
| Length of ICU stay, day (median) |
7 |
5.3-11.5 |
| 28-day survivor, n |
9 |
90.00% |
| Condition on sleep measurement |
|
|
| Day of measurement (ICU admission=1) (median) |
4.5 |
4.0-5.8 |
| MV with intubation, n |
3 |
30.00% |
| Sedatives, n |
1 (Dexmedetomidine) |
|
| Fentanyl, n |
5 |
|
| Ramelteon, n |
1 |
|
| Suvorexant, n |
4 |
|
| Maximum RASS (median) |
|
|
| Day (06:00-20:59) |
-0.5 |
−1.0-0.0 |
| Night (21:00-05:59) |
−1 |
−1.0-−0.3 |
| Delirium, n |
3 |
30 |
Note: MV: Mechanical Ventilation; RASS: Richmond Agitation Sedation Scale
Table 1: Demographic and clinical characteristics of study patients
| |
Median |
Interquartile range |
| 24 hour PSG distribution |
| TST (min) |
929.5 |
662.5-951.0 |
| SE (%) |
64.5 |
46.0-66.0 |
| WK (%) |
35.5 |
34.0-51.6 |
| N1 (%) |
22.2 |
15.3-37.6 |
| N2 (%) |
27.5 |
15.1-30.8 |
| N3 (%) |
1.3 |
0.0-6.1 |
| REM (%) |
0 |
0.0-0.3 |
| Nighttime PSG distribution (21:00-05:59) |
| TST (min) |
464.5 |
448.5-518.0 |
| SE (%) |
86 |
83.1-95.9 |
| WK (%) |
14 |
4.1-16.9 |
| N1 (%) |
22.2 |
14.7-35.2 |
| N2 (%) |
28.9 |
20.1-57.7 |
| N3 (%) |
0.5 |
0.0-5.9 |
| REM (%) |
0 |
0.0-0.7 |
| Arousal Index (arousals/hr) |
9.5 |
7.8-20.1 |
| Frequency of sleep stage shift (/hr) |
7.8 |
2.9-10.1 |
| PSI (427075 2 seconds epochs) for each sleep stage |
| WK |
87 |
84.0-90.0 |
| N1 |
86 |
64.0-88.0 |
| N2 |
42 |
24.0-86.0 |
| N3 |
21 |
19.0-24.0 |
| REM |
32 |
28.0-38.8 |
| RCSQ |
|
|
| Average of all 5 questions |
71 |
34.4-88.8 |
| Sleep depth |
62 |
38.0-89.0 |
| Sleep latency |
78 |
61.0-82.0 |
| Frequency of awakenings |
60 |
22.0-87.0 |
| Sleep latency after awakenings |
89 |
25.0-92.5 |
| Sleep quality |
82 |
28.0-91.0 |
Note: PSG: Polysomnography, PSI: Patient State Index, RCSQ: Richards-Campbell Sleep Questionnaire, TST: Total Sleep Time (N1+N2+N3+REM), SE: Sleep Efficiency (TST/1440 for 24 hour, TST/540 for night time), WK: Wake, N1: Sleep stage N1, N2: Sleep Stage N2, N3: Sleep Stage N3, REM: Rapid Eye Movement Sleep, Arousal Index: Number of arousals per hour, Frequency of sleep stage shift: Number of transitions per hour within Wake, REM, and NREM periods (NREM: Non-Rapid Eye Movement Sleep; N1+N2+N3)
Table 2: Results of PSG, PSI, and RCSQ
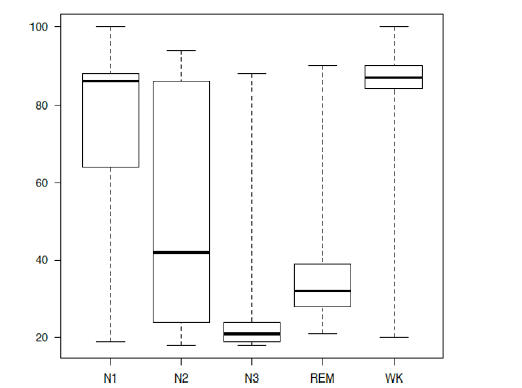
Figure 2: The boxplot of PSI compared to each stage from PSG N1=Sleep stage N1, N2=Sleep stage N2, N3=Sleep stage N3, REM=Rapid Eye Movement, WK=Wake, the horizontal line=sleep stages of PSG, the vertical line=PSI, The boxes show the 25% to 75% interquartile range with the median (thick line inside) and the whiskers indicate the maximum and the minimum values.
We found significant correlations between the total RCSQ scores and (1) night time stage N2 from PSG (r=0.741, 95% CI from 0.209 to 0.935), (2) PSG stage N2 combined with N3 and REM (r=0.801, 95% CI from 0.347 to 0.951), and (3) nighttime average PSI (r=-0.816, 95% CI from -0.955 to -0.383) (Table 3). We additionally analyzed and provide the Receiver Operating Characteristic (ROC) curve of the PSI in Figure 3, indicating the threshold necessary to distinguish between stage N1 and N2 as well as the respective high specificity, sensitivity, and the Area under the Curve (AUC). The results indicate the sleep depth bordering stage N1 and N2 to be 67.0 (Specificity, 0.641; Sensitivity, 0.845) and the AUC is 0.818 (Figure 3).
| PSG night time |
|
Coefficient [95% CI] |
P-value |
| SE |
0.492 [-0.199 0.856] |
0.148 |
| 0.398 [-0.309 0.822] |
0.255 |
| N1 |
-0.533 [-0.870 0.146] |
0.113 |
| -0.515 [-0.864 0.169] |
0.127 |
| N2 |
0.741 [0.209 0.935] |
0.014* |
| 0.803 [0.352 0.952] |
0.005* |
| N3 |
0.169 [-0.515 0.722] |
0.64 |
| -0.044 [-0.655 0.603] |
0.905 |
| REM |
0.432 [-0.271 0.835] |
0.212 |
| 0.301 [-0.406 0.782] |
0.399 |
| N1+N2 |
0.298 [-0.408 0.781] |
0.403 |
| 0.369 [-0.340 0.810] |
0.295 |
| N2+N3+REM |
0.801 [0.347 0.951] |
0.005* |
| 0.710 [0.146 0.926] |
0.021* |
| N3+REM |
0.214 [-0.480 0.743] |
0.553 |
| -0.014 [-0.638 0.621] |
0.969 |
| Arousal index |
-0.026 [-0.645 0.614] |
0.943 |
| -0.077 [-0.674 0.581] |
0.832 |
| Frequency of sleep stage shift |
-0.211 [-0.742 0.483] |
0.559 |
| -0.099 [-0.686 0.566] |
0.785 |
| PSI night time average |
-0.816 [-0.955 -0.383] |
0.004* |
| -0.700 [-0.923 -0.126] |
0.024* |
Note: Upper=RCSQ-average, Lower=RCSQ-depth, *=P<0.05; RCSQ: Richards-Campbell Sleep Questionnaire, PSG: Polysomnography, SE: Sleep Efficiency (TST/1440 for 24 hour, TST/540 for night time, TST: Total Sleep Time), N1: Sleep Stage N1, N2: Sleep Stage N2, N3: Sleep Stage N3, REM: Rapid Eye Movement Sleep, Arousal Index: Number of arousals per hour, Frequency of sleep stage shift: Number of transitions per hour within Wake, REM, and NREM periods (NREM: Non-Rapid Eye Movement Sleep; N1+N2+N3), PSI: Patient State Index
Table 3: Pearson’s correlation among sleep parameters, the total RCSQ, and the RCSQ sleep depth subscale
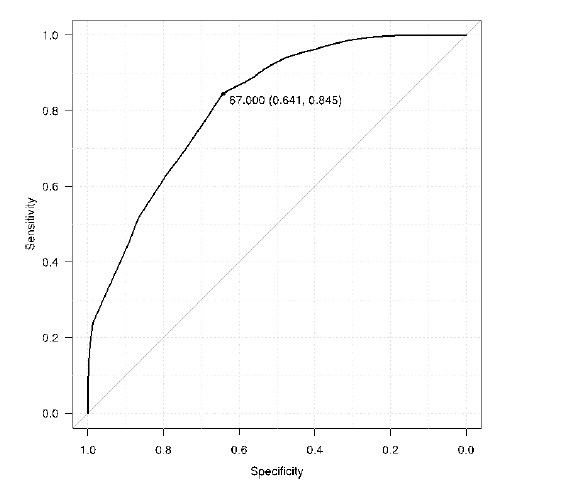
Figure 3: ROC curve of PSI to sleep depth with stage N2 or deeper PSI: Patient State Index Cutoff value of PSI is 67.0 (Specificity, 0.641; Sensi- tivity, 0.845) and the area under the curve 0.818
Discussion
The main findings from our study highlight a progressive decrease in PSI along with sleep depth based on PSG data. There was also a significant correlation of PSI with the ratio of the stage N2 and RCSQ for nighttime sleep. Sleep assessment was easily conducted using SedLine, with the exception of several artifacts in a single case. Importantly, the frequency of patients’ refusal or complaints was much higher with PSG than with SedLine. Our assessment of sleep architecture in the 24 hour PSG data described an increase in light sleep and a reduction of restorative sleep in critically ill patients, despite preserved quantity of nighttime sleep. Our findings match those previously reported [1-4]. Anderson et al. showed that the sleep architecture among those in the critical care settings included 11% to 59% of stage N1, 26% to 74% of stage N2, 0.15% to 22% of stage N3, and 1% to 12% of REM [5]. Widely varying ranges among patients, as well as among studies, are likely to reflect the heterogeneity of this clinical population in respect to differences in diseases, disease severity, or medications [5]. Although most patients in our small study population were postoperative cardiovascular patients and their sleep measurements were conducted appropriately on the 4th or 5th day after ICU admission, the PSG results suggest high inter individual differences between conditions. PSI decreased along with sleep depth, but its distribution was not equal between sleep stages.
The PSI values between WK and stage N1 were quite close compared to those from stage N3 and REM, whereas the wide distribution of stage N2 largely overlapped with the PSI values of stage N1 and REM. A previous study measuring sleep depth in healthy subjects with BIS produced similar results, but values of REM presented distributions closer to the ones observed during stage N1 [17]. The overlap between consecutive sleep stages could also be explained by the continuity of the sleep process, with soft transitions between sleep stages [17]. Vacas et al. reported that SedLine seemed to identify WK or stage N2 when PSG would label the same as stage N1 [16]. Further, atypical PSG signs in critically ill patients, such as a lack of K complex or spindles that are typically indicative of stage N2, could not be distinguished by SedLine [4]. RCSQ values describing sleep satisfaction also showed wide variance among subjects in the present study. Similar to the PSG results, this may reflect inter individual differences from diverse medical conditions. Importantly, RCSQ showed a significant positive correlation with the nighttime ratio of stage N2 alone or combined with stage N3 and REM as well as a negative correlation with the nighttime average PSI. A significant correlation was also found for one of the five RCSQ subscales assessing sleep depth. Subjective sleep evaluation is considered to depend on sleep depth, as suggested by Richards et al. [12].
In the present study, RCSQ was found to correlate with stage N2 alone or combined with N3 and REM. Since most of the sleep time recorded in our study consisted of epochs scored as stage N1 or N2 and restorative sleep was strongly reduced, RCSQ results suggest that satisfactory sleep could be achieved with adequate time in stage N2. In previous studies comparing BIS and PSG, stage N1 and N2 were combined in a broad category of light sleep [17]. However, we separated stages N1 and N2 because subjective feelings of good sleep were positively correlated with time spent in stage N2 or with N3, REM, or both N3 and REM in our study. This is the main reason why we analyzed and provide the ROC of PSI in Figure 3, indicating the threshold necessary to distinguish between stage N1 and N2 as well as the respective high specificity, sensitivity, and AUC. The cutoff value of PSI can be interpreted as a target for sleep depth to fulfill subjective sleep quality rather than detecting sleep or awake states. The specificity may not have been as high as the sensitivity due to the overlap between consecutive sleep stages and the potential difficulty in identifying stage N2 in the critical population, as already mentioned. Nevertheless, the precise quantity requirements of each sleep stage could not be identified in our study.
Limitations
Some study limitations should be acknowledged. First, our sample size was small and resulted from recruitment at a single treatment center. This restricts our ability to generalize our results. Although more than 400,000 components of data were analyzed to validate PSI against PSG, the correlation between objective and subjective sleep evaluation was based on the data of only 10 subjects. We should also consider the interacting influence of the processed EEG monitors in the ICU that are different from those typically applied during general anesthesia [20]. As the heterogeneity among the 10 study participants also limits the data, future multi center studies with larger sample sizes and a variety of critical care patients may provide a clearer picture. Importantly, the sleep measurement devices should be validated in healthy subjects as well. Second, the accuracy of the PSG scoring was not verified in this study. The small study population included 3 delirious patients, and several patients were treated with small doses of fentanyl or dexmedetomidine, with or without mechanical ventilation. It has been previously reported that restorative sleep is reduced in delirious patients or opiate users [21]. Moreover, an increase in stage N3 with slow waves is commonly observed in cases of encephalopathy [6,22-24].
Dexmedetomidine could then improve SE, increasing the amounts of stage N2 relative to stage N1, but without increasing restorative sleep [25]. Regardless of delirium, mechanical ventilation, or sepsis, various atypical PSG findings, including the absence of particular waveforms in stage N2 or the discrepancy between behavioral assessment and PSG staging (socalled “pathological wakefulness”), are detectable in critically ill patients [6,23,24]. However, when, why, or how often these may happen remains unknown. Additionally, due to these abnormalities, the appropriateness of PSG scoring using the standard classification is suspicious in the context of the ICU [4,6,7,24,26]. A modified classification has been proposed, but has not yet been validated [4,7,22,24,26]. The same concerns may apply to PSI, since it is converted from EEG signals that are used to evaluate the depth of sedation. For this reason, comparing objective and subjective sleep metrics as well as verifying their association is necessary. The third limitation of this study is that factors potentially affecting subjective sleep evaluation could not be precisely identified, although our results suggest that a sleep depth of stage N2 or deeper is required for sleep satisfaction. Importantly, we found PSI to be related to sleep depth and to allow the discrimination between sleep depth degrees with high sensitivity and specificity. However, not only sleep depth was identified to be important for subjective sleep quality, but also sleep quantity at each depth. Yet, the length of deeper sleep that is necessary to achieve satisfactory sleep quality is still unclear. Frequent sleep fragmentation, commonly observed in critical care settings, was unremarkable in our results. The effects of sleep continuity on sleep quality as well as the required quantity of deep sleep should be further investigated. Finally, stage N2 sleep produced subjective sleep satisfaction, but this alone may not be sufficient for truly good sleep. The most important domain of sleep to maintain physical or psychological wellbeing is the restorative sleep obtained during stage N3 and REM, which was highly impaired in our patients. Thus, further validation should be conducted in healthy populations with normal sleep architectures. Further investigation with a larger cohort of patients is needed.
Conclusion
This preliminary investigation showed that PSI, provided by SedLine measurements, was distributed in accordance with the sleep depth based on PSG data. It also showed an association with subjective sleep quality, evaluated using the RCSQ, and with PSG parameters. Root® with SedLine®, a portable EEG monitor, is a feasible sleep monitoring device for critically ill patients, predicting subjective sleep satisfaction. These findings could further help in enhanced patient care through ease of monitoring and understanding the sleep quality in such patients.
Author's Contribution
KN and MS formulated the study concept and design. KN and TM acquired the data. KN and TM performed statistical analysis. KN, MS, and TM conducted the analysis and interpretation of data. KN drafted the manuscript. MS, TM, YI, and JS performed the critical revision of the manuscript for important intellectual content. MS supervised the study. All authors have read and approved final manuscript and take response for the integrity of the data and the accuracy of the data analysis.
Acknowledgement
The authors acknowledge Naoto Yoshida and Satoko Yagihashi, registered nurses, for their assistance in the implementation of PSG, as well as Editage [http://www.editage.com] for editing and reviewing this manuscript for English language.
Conflict of Interest
The authors declare that they have no conflict of interests.
Ethics Approval and Consent to Participate
This observational study was part of an interventional trial approved by the Institutional Review Board of Jichi Medical University Saitama Medical Center. Informed consent with written confirmation was obtained from patients (or their family when the patient was unable to sign). Only anonymized data were entered into the study database and used for analysis.
Consent for Publication
Not applicable.
Availability of Data and Materials
The datasets used during the current study are available by the corresponding author on reasonable request.
Funding
The authors received Grant Aid for Scientific Research C from the Japan Society for the Promotion of Science (16K11418).
References
- Beltrami FG, Nguyen XL, Pichereau C, Maury E, Fleury B, et al. (2015) Sleep in the intensive care unit. J Bras Pneumol 41(6): 539-546.
[Crossref] [Google Scholar] [PubMed]
- Weinhouse GL, Schwab RJ (2006) Sleep in the critically ill patient. Sleep 29(5): 707-716.
[Crossref] [Google Scholar] [PubMed]
- Delaney LJ, Van Haren F, Lopez V (2015) Sleeping on a problem: The impact of sleep disturbance on intensive care patients-a clinical review. Ann Intensive Care 5: 3.
[Crossref] [Google Scholar] [PubMed]
- Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, et al. (2015) Sleep in the intensive care unit. Am J Respir Crit Care Med 191(7): 731-738.
[Crossref] [Google Scholar] [PubMed]
- Andersen JH, Boesen HC, Skovgaard Olsen K (2013) Sleep in the intensive care unit measured by polysomnography. Minerva Anestesiol 79(7): 804-815.
[Google Scholar] [PubMed]
- Drouot X, Cabello B, d'Ortho MP, Brochard L (2008) Sleep in the intensive care unit. Sleep Med Rev 12(5): 391-403.
[Crossref] [Google Scholar] [PubMed]
- Rittayamai N, Wilcox E, Drouot X, Mehta S, Goffi A, et al. (2016) Positive and negative effects of mechanical ventilation on sleep in the ICU: A review with clinical recommendations. Intensive Care Med 42(4): 531-541.
[Crossref] [Google Scholar] [PubMed]
- Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, et al. (2012) Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders' conference. Crit Care Med 40(2): 502-509.
[Crossref] [Google Scholar] [PubMed]
- Kamdar BB, Needham DM, Collop NA (2012) Sleep deprivation in critical illness: Its role in physical and psychological recovery. J Intensive Care Med 27(2): 97-111.
[Crossref] [Google Scholar] [PubMed]
- Bourne RS, Minelli C, Mills GH, Kandler R (2007) Clinical review: Sleep measurement in critical care patients: Research and clinical implications. Crit Care 11(4): 226.
[Crossref] [Google Scholar] [PubMed]
- Richards K (1987) Techniques for measurement of sleep in critical care. Focus Crit Care 14(4): 34-40.
[Google Scholar] [PubMed]
- Richards KC, O’Sullivan PS, Phillips RL (2000) Measurement of sleep in critically ill patients. J Nurs Meas 8(2): 131-144.
[Crossref] [Google Scholar] [PubMed]
- Hoey LM, Fulbrook P, Douglas JA (2014) Sleep assessment of hospitalised patients: A literature review. Int J Nurs Stud 51(9): 1281-1288.
[Crossref] [Google Scholar] [PubMed]
- Frisk U, Nordström G (2003) Patients' sleep in an intensive care unit patients' and nurses' perception. Intensive Crit Care Nurs 19(6): 342-349.
[Crossref] [Google Scholar] [PubMed]
- Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, et al. (2017) AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med 13(5): 665-666.
[Crossref] [Google Scholar] [PubMed]
- Vacas S, McInrue E, Gropper MA, Maze M, Zak R, et al. (2016) The feasibility and utility of continuous sleep monitoring in critically ill patients using a portable electroencephalography monitor. Anesth Analg 123(1): 206-212.
[Crossref] [Google Scholar] [PubMed]
- Murata H, Oono Y, Sanui M, Saito K, Yamaguchi Y, et al. (2019) The Japanese version of the Richards−Campbell sleep questionnaire: Reliability and the validity. Nurs Open 6(3): 808-814.
[Crossref] [Google Scholar] [PubMed]
- Giménez S, Romero S, Alonso JF, Mañanas MÁ, Pujol A, et al. (2017) Monitoring sleep depth: Analysis of bispectral index (BIS) based on polysomnographic recordings and sleep deprivation. J Clin Monit Comput 31(1): 103-110.
[Crossref] [Google Scholar] [PubMed]
- Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3): 452-458.
[Crossref] [Google Scholar] [PubMed]
- Dahaba AA (2019) Thinking outside the box. Off-label use of bispectral Index within context and limitations for conditions other than depth of anesthesia. Minerva Anestesiol 85(2): 189-193.
[Crossref] [Google Scholar] [PubMed]
- Watson PL, Ceriana P, Fanfulla F (2012) Delirium: Is sleep important? Best Pract Res Clin Anaesthesiol 26(3): 355-366.
[Crossref] [Google Scholar] [PubMed]
- Watson PL, Pandharipande P, Gehlbach BK, Thompson JL, Shintani AK, et al. (2013) Atypical sleep in ventilated patients: Empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med 41(8): 1958-1967.
[Crossref] [Google Scholar] [PubMed]
- Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, et al. (2000) Sleep in critically ill patients requiring mechanical ventilation. Chest 117(3): 809-818.
[Crossref] [Google Scholar] [PubMed]
- Boesen HC, Andersen JH, Bendtsen AO, Jennum PJ (2016) Sleep and delirium in unsedated patients in the intensive care unit. Acta Anaes Scandinavica 60(1): 59-68.
[Crossref] [Google Scholar] [PubMed]
- Alexopoulou C, Kondili E, Diamantaki E, Psarologakis C, Kokkini S, et al. (2014) Effects of dexmedetomidine on sleep quality in critically ill patients: A pilot study. Anesthesiology 121(4): 801-807.
[Crossref] [Google Scholar] [PubMed]
- Drouot X, Roche-Campo F, Thille AW, Cabello B, Galia F, et al. (2012) A new classification for sleep analysis in critically ill patients. Sleep Med 13(1): 7-14.
[Crossref] [Google Scholar] [PubMed]
Citation: Nagatomo K, Sanui M, Masuyama T, Iizuka Y, Shiotsuka J (2023) A Preliminary Study on the Utility of Root® with SedLine® for Sleep Evaluation in Critically Ill Patients: Comparison with Polysomnography and the Richards-Campbell Sleep Questionnaire. J Intensive Crit Care. 9:012.
Copyright: © 2023 Nagatomo K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.




