Sharanpreet Kaur*, Megan Sidana, Diandra Ruidera and Xolani Mdluli
Department of Internal Medicine, Desert Regional Medical Center, Palm Springs, CA, USA
- *Corresponding Author:
- Sharanpreet Kaur
Department of Internal Medicine, Desert Regional Medical Center, Palm Springs, CA, USA
Tel: +4087058450
E-mail: skaur@atsu.edu
Received Date: July 16, 2019; Accepted Date: August 19, 2019; Published Date: August 27, 2019
Citation: Kaur S, Sidana M, Ruidera D, Mdluli X (2019) A Unique Treatment Approach for a Fulminant Case of Clostridioides difficile Infection. J Infec Dis Treat Vol.5 No.1:2.
Keywords
Clostridioides difficile infection; Fulminant Clostridioides difficile infection; Fidaxomicin metronidazole
Introduction
Clostridioides (formerly known as Clostridium) difficile infection (CDI) is one of the most common and severe hospital-acquired infections affecting the gastrointestinal tract [1,2]. The first case of CDI was reported in 1935 in an infant by Hall and O’Toole [3]. In 1978, a group of scientists discovered that the majority of the antibiotic-associated diarrhea cases were caused by C. difficile [4]. Per author Bartlett, many earlier studies reported Clindamycin as the most likely antibiotic causing the C. difficile infections [5]. From 1983 to 2003 cephalosporins were noted to be the predominant antibiotic correlated with CDI (5). A study conducted in 2013 by Brown et al., concluded that certain antibiotics such as Clindamycin, carbapenems and fluoroquinolones were most associated with causing CDI [6]. The necessary use of these antibiotics can lead to the cure of one infection but can also potentially cause other severe infections.
Despite guideline-based therapy, fulminant cases of CDI have continued to develop and cause significant morbidity and mortality, causing almost half a million infections and approximately 29,000 deaths in 2011 [7]. Per the Center for Disease Control, one in eleven patients over the age of 65 diagnosed with a healthcare-associated CDI dies within a month [8]. According to 2016 study by Zhang et al., the total annual CDI attributable cost in the United States was roughly 6.3 billion dollars [9]. An article by Bouza reported that recurrent cases of CDI can cost more than the initial infection [10]. Measures to prevent CDI recurrence can help minimize financial expenses, and therefore are integral to both cost savings and prevention of readmissions. Dallal et al. explained in their article that clinical symptoms of CDI can range from diarrhea, to septic shock and death, and can lead to life threatening systemic toxicity despite appropriate therapy [11].
Although the Infectious Diseases Society of America (IDSA) provides treatment guidelines for fulminant CDI cases, physicians frequently encounter cases which are unresponsive to guideline-based therapy. We present a case where the guideline-based recommendation was unsuccessful, and the treatment regimen was altered to yield a successful outcome for our patient.
Case Report
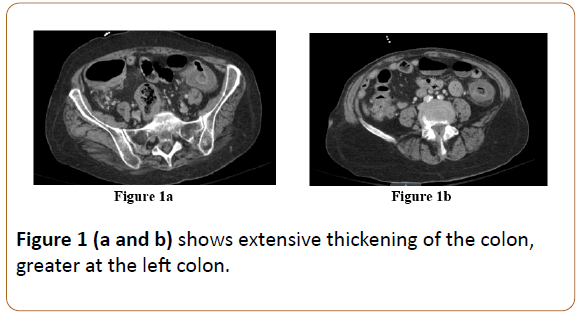
We present the case of a 71-year-old female with a past medical history of Wegener's granulomatosis, ischemic stroke and eight episodes of CDI over the past two years with two failed attempts of fecal transplants. She had been seen by an infectious diseases specialist outpatient for her extensive history of recurrent CDI. The patient presented to the ED with a chief complaint of fever, abdominal pain, weakness and watery diarrhea which had been ongoing for one day. Upon presentation to the ED, the patient’s vitals were significant for temperature of 38.5°C, heart rate of 105 beats per minute and blood pressure of 87/46. Her labs were significant for white blood cell (WBC) count of 32.3, ANC of 29.3 and lactic acid of 2.66. Other lab values were within normal limits. A CT scan of the abdomen was obtained which was consistent with colitis and demonstrated extensive thickening of the colon, specifically the left colon (Figures 1a and 1b).
Figure 1 (a and b) shows extensive thickening of the colon, greater at the left colon.
The patient was febrile, tachycardic, and had significant leukocytosis with poor response to fluid resuscitation. She was therefore noted to be in septic shock and admitted to the ICU. Due to her hemodynamic instability, she was started on norepinephrine. Her underlying source of sepsis was assumed to be CDI, and C. difficile PCR and stool cultures were ordered. At that point, the infectious diseases team was consulted and recommended a regimen of vancomycin 500 mg oral every 6 hours, vancomycin 500 mg rectal every 6 hours, and metronidazole 500 mg intravenously (IV) three times a day. Her C. difficile PCR returned positive and despite appropriate therapy, the patient continued to have diarrhea and an up-trending leukocytosis. Furthermore, due to her profuse diarrhea, it was unclear if she was absorbing the rectal vancomycin, so the rectal vancomycin was discontinued after four days of triple therapy. The patient was continued on oral vancomycin and IV metronidazole. Her vitals began to stabilize, and leukocytosis began down trending. However, four days later, the patient was noted to have a rise in WBC. The decision was then made to stop oral vancomycin and start fidaxomicin 200 mg orally twice a day and continue IV metronidazole 500 mg three times a day. Within four days, the patient ’ s leukocytosis had down trended to normal and she began having more solid and formed stools. Overall the patient began to clinically improve. She finished a 10-day course of oral fidaxomicin and IV metronidazole, and a customized vancomycin taper regimen was created for the patient upon discharge.
Discussion
This particular case highlights the difficulty physicians worldwide frequently encounter in fulminant CDI cases unresponsive to guideline-based therapy. Several studies have been conducted and lend to comparative analyses. In a study published in the New England Journal of Medicine in 2015, 453,000 cases of CDI were reported in 2011 which included 29,300 deaths [7]. Another report by Hall et al. demonstrated C. difficile, along with Norovirus, was one of the emerging causes of gastroenteritis associated deaths between the years of 1999 and 2007 [12]. The morbidity and mortality secondary to CDI is high and the cost of CDI treatments is a huge burden on the healthcare system in the United States. Therefore, reporting cases such as these with successful alternative treatments for fulminant CDI cases can guide other medical providers in refractory cases.
The current IDSA guidelines recommend starting patients with fulminant CDI on vancomycin 125 mg orally four times a day with metronidazole 500 mg IV three times a day plus optional vancomycin 500 mg rectal every 6 hours. In our case, the patient was started on this recommended regimen. In order to measure its efficacy, laboratory values, vitals and fecal outputs were closely monitored. A few days after initiating the therapy the patient was found to be unresponsive to the treatment, leading to alteration in her treatment regimen.
Per literature review, other cases were also found where alternative treatments were applied to best serve the patient. In a case reported by Arnott et al., their team administered fidaxomicin through a mucous fistula, which saved the patient from requiring a total colectomy [13]. In a study conducted by Johnson et al., patients who had anywhere from four to eight recurrent CDI episodes and had completed 14 days of oral vancomycin were given 400 to 800 mg of rifaximin for 2 weeks. Eighty eight percent of the patients participating in the trial had no further recurrent CDI episodes [14]. Exclusive probiotic use also warrants mention. In a study conducted by McFarland et al., the addition of probiotic Saccharomyces boulardii to the standard antibiotics lowered the recurrence rate by 35% when used in patients with recurrent CDI [15]. These cases illustrate that rifaximin and probiotic use can further enhance a vancomycin taper regimen. However, these approaches were not explored in our patient.
Per literature review, certain risk factors for developing fulminant CDI include prior history of CDI, hospital admission, antibiotic use, advancing age, immunosuppression, and solid organ transplants particularly lung transplants [16]. Dallal et al. explain that due to potential rejection, lung transplant recipients need frequent immunosuppression and those with recurrent respiratory infections require necessary antibiotics [11]. Hence, both scenarios have been found to lead to increased instances of fulminant CDI [11]. This demonstrates that in addition to antibiotic exposure, impairment in immune status can fuel recurrent CDI development.
Conclusion
Despite an established treatment protocol for fulminant CDI set forth by IDSA, our patient required an alternate regimen. Clinicians often find that regardless of guidelines, they must continuously develop their treatment strategies to best accommodate the patient’s evolving symptoms. Although our patient’s presenting symptoms and clinical course were very similar to those observed in patients with fulminant CDI, her minimal response to guideline-based therapy made treatment challenging. Therefore, a unique regimen of fidaxomicin 200 mg orally twice a day with IV metronidazole 500 mg three times a day was attempted for our patient. After a successful resolution of symptoms on a 10-day course of this combination, she was discharged on a customized vancomycin taper in order to help prevent further recurrences of CDI. The collaborative effort used to determine a novel treatment regimen for our patient was integral to successful resolution of her infection.
References
- Heinlen L, Ballard JD (2010) Clostridium difficile infection. Am J Med Sci 340: 247-252.
- Zilberberg, Marya D (2008) Increase in Adult Clostridium Difficile–Related Hospitalizations and Case-Fatality Rate, United States, 2000-2005. Emerging Infectious Diseases 14: 929-931.
- Hall IC, O’Toole E (1935) Intestinal flora in new-born infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child 49: 390-402.
- George WL, Sutter VL, Goldstein EJ (1978) Aetiology of antimicrobial-agent associated colitis. Lancet 1: 802-803.
- Bartlett JG (2006) Narrative review: The new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med 145: 758-764.
- Brown KA, Khanafer N, Daneman N, Fisman DN (2013) Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother 57: 2326-2332.
- Lessa FC, Mu Y, Bamberg W (2015) Burden of Clostridium difficile infection in the United States. New England Journal of Medicine 372: 2368-2370.
- https://www.cdc.gov/cdiff/what-is.html.
- Zhang S, Palazuelos-Munoz S, Balsells EM (2016) Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis 16: 447.
- Bouza E (2012) Consequences of Clostridium difficile Infection: Understanding the Healthcare Burden. Clinical Microbiology and Infection 18: 5-12.
- Dallall RM, Harbrecht BG, Boujoukas AJ (2001) Fulminant Clostridium difficile: An Underappreciated and Increasing Cause of Death and Complications. Annals of Surgery 235: 363-372.
- Aron JH, Aaron TC, Clifford McDonald L, Umesh DP, Ben AL (2012) The Roles of Clostridium difficile and Norovirus Among Gastroenteritis-Associated Deaths in the United States, 1999–2007. Clinical Infectious Diseases 55: 216-223.
- Arnott S, Skancke M, Chen S, Abell B (2018) A case report of successful management of Clostridium difficile colitis with antegrade fidaxomicin through a mucous fistula obviating the need for subtotal colectomy. International Journal of Surgery Case Reports 42: 79-81.
- Johnson S, Screiever C, Galang M, Kelly CP, Gerding DN (2007) Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with Vancomycin and Rifaximin. Clinical Infectious Disease 44: 846-848.
- McFarland LV, Surawicz CM, Greenberg RN (1994) A randomized placebo controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 271: 1913-1918.
- Kelly CP (2001) A 76-year-old man with recurrent Clostridium difficile-associated diarrhea. JAMA 301: 954-962.