- (2010) Volume 11, Issue 6
Serkan Ocal1, Haldun Selçuk1, Murat Korkmaz1, Hakan Unal2, Ugur Yilmaz1
1Department of Gastroenterology, Baskent University. Ankara, Turkey
2Department of Gastroenterology, Baskent University. Istanbul, Turkey
Received July 11th, 2010 - Accepted August 9th, 2010
Context Drugs are related to the etiology of acute pancreatitis in approximately 1.4-2.0% of cases. Although antibiotics constitute a small number of the drugs suspected, tetracycline is the most encountered antibiotic among those drugs. Case report A 33-year-old woman was admitted to the emergency room complaining of nausea and abdominal pain after the use of doxycycline 500 mg and ornidazole 500 mg twice daily for three days for a vaginal infection. She experienced epigastric pain, which worsened over time and radiated to her back. After a detailed evaluation, she was diagnosed as having mild acute pancreatitis which improved with medical treatment. All other possible causes of pancreatitis were eliminated. Conclusions Antibiotic-associated pancreatitis usually has a silent and mild course. To the best of our knowledge the literature reports only two cases of doxycycline-induced acute pancreatitis reported and there are no reports of pancreatitis associated with ornidazole. Our case is the first case reported in which doxycycline and ornidazole coadministration induced acute pancreatitis.
Drug toxicity; Pancreatitis /etiology
The frequent use of multiple drugs increases the risk of drug-induced pancreatitis [1, 2, 3, 4]. Detailed information regarding antibacterial drug-induced acute pancreatitis published in 2005 reported that 34 cases of them were related to tetracycline, 24 cases to trimethoprim/sulfamethoxazole, 25 cases to rifampin , 11 cases to erythromycin and sporadic cases were induced by metronidazole, isoniazid and amoxicillinclavulanic acid [4]. There were two cases of doxycycline-induced acute pancreatitis [3, 5]. We do not have additional information about the course of the disease and the final status of the patient. We could not find any cases of ornidazole-induced pancreatitis in literature. Our case is the first with detailed patient information regarding doxycycline- and/or ornidazoleinduced acute pancreatitis.
A 33-year-old female patient was admitted to the emergency room complaining of nausea and abdominal pain after the use of doxycycline 500 mg and ornidazole 500 mg twice daily for three days for a vaginal infection. She experienced abdominal pain in the epigastric region which radiated to her back and worsened with time.
Upon admittance to the emergency room, she had stable vital signs. Her body temperature was 36.7°C, blood pressure 120/80 mmHg and pulse rate was 88 min-1. She experienced pain during epigastric palpation and had slightly diminished bowel sounds. Her physical examination was otherwise normal.
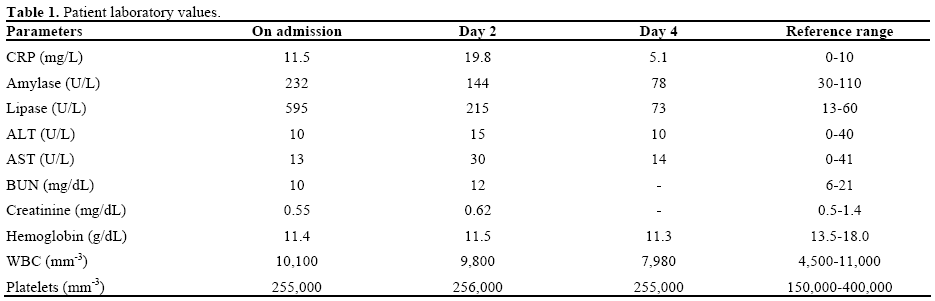
Her laboratory values are shown in detail in Table 1. Serum liver and renal function values were all in the normal range with elevated serum amylase and lipase levels: 220 U/L (reference range: 30-110 U/L) and 595 U/L (reference range: 13-60 U/L), respectively. Serum total cholesterol and triglyceride levels were 128 mg/dL (reference range: 130-200 mg/dL) and 130 mg/dL (reference range: 50-160 mg/dL), respectively. She had normal levels of serum calcium (8.8 mg/dL; reference range: 8.5-10.5 mg/dL) and total bilirubin (0.3 mg/dL; reference range: 0.2-1.2 mg/dL) with a slightly elevated CRP level (11.5 mg/L; reference range: 0-10 mg/L). She had no signs of viral infection, no history of trauma or alcohol use and no family history of pancreatic diseases.
Upper abdominal ultrasonography revealed normal images of the pancreas, liver, gallbladder and intraextrahepatic bile ducts. The pancreas was also assessed as normal by abdominal-computed tomography. The ornidazole and doxycycline were discontinued and supportive medical treatment was begun. Her symptoms decreased after the first day and were completely resolved after two days. Her serum amylase, lipase and CRP levels returned to normal after three days.
Drug-induced pancreatitis constitutes 1.4% of all causes of acute pancreatitis. Mallory and Kern [6] developed diagnostic criteria for evaluating druginduced pancreatitis in 1980. These criteria can be summarized as: 1) the pancreatitis develops during drug therapy; 2) all other possible causes of pancreatitis should be eliminated; 3) the pancreatitis should resolve after discontinuing the suspected drug; and 4) the re-appearance of pancreatitis after using the same drug. Drug induced pancreatitis can be classified as definite, highly probable or weakly probable in accordance with those criteria.
In our patient, the acute pancreatitis developed three days after the administration of doxycycline and ornidazole and was resolved three days after discontinuation of the drugs. All other possible factors, such as alcohol use, gallstones, hypercalcemia, hyperlipidemia, family history and malignancy were eliminated by means of physical examination, blood chemistry tests and radiodiagnostic imaging modalities. The period between the start of the antibiotic therapy and the development of acute pancreatitis is variable and has been reported as 1-37 days for metronidazole [7], the 5th day of clarithromycin use [8] and immediately after isoniazid use [9]. Drug-induced pancreatitis developed on the third day of antibiotic use in our patient. As reported previously, our patient experienced a mild course of acute pancreatitis, which was resolved spontaneously with supportive care in a few days.
Other predictable adverse effects of doxycycline are the elevation of liver and renal function tests, diarrhea, nausea, vomiting and dysphagia while ornidazole has prominent neurologic side effects. Doxycycline is part of the tetracycline group of antibiotics. One case of demeclocycline [10] and four cases of minocycline [11, 12], also members of the tetracycline group, have been linked to acute pancreatitis cases. More interestingly, Papaioannides et al. reported a case of brucellosisinduced acute pancreatitis which was resolved after doxycycline treatment [13]. It is well known that pancreatic phospholipase A2 contributes to the tissue damage associated with acute pancreatitis. Since, in experimental models, lipophilic tetracyclines, such as minocycline and doxycycline, are anti-inflammatory, Pruzanski et al. examined their effects on pancreatic phospholipase A2 activity using two assay systems in vitro [14]. They found that minocycline and, to a lesser degree, doxycycline were markedly inhibitory to both pancreatic and non-pancreatic phospholipase A2. The possible protective mechanism of doxycycline in acute pancreatitis may be elucidated by inhibiting pancreatic phospholipase A2.
We did not recognize any abnormalities related to acute pancreatitis, such as pancreatic edema or peripancreatic fluid, during radiologic investigations. The only reported doxycycline-induced acute pancreatitis case did not have any data regarding laboratory or radiologic imaging while two of the seven metronidazole-induced acute pancreatitis cases revealed radiologic abnormalities [7]. Steinberg and Tenner initially classified drug-induced pancreatitis as associated with intrinsic toxins versus idiosyncratic reactions following the well-known classification of drug-induced liver diseases [1]. The authors hypothesized that the direct toxic effect of free oxygen radicals on pancreatic beta cells, and immunologic and metabolic injury of the pancreatic duct could be the possible mechanisms of metronidazole-induced acute pancreatitis [7]. We did not recognize any data in the literature regarding the mechanisms of how doxycycline and ornidazole cause acute pancreatitis.
In conclusion, we think that our case is unique and interesting because it is probably the first case of doxycycline- and/or ornidazole-induced acute pancreatitis. Although it is likely that this patient suffered from acute pancreatitis due to doxycycline and ornidazole since other causes of pancreatitis were eliminated, it is difficult to conclude which drug was the main culprit since re-challenge is not ethical and might harm the patient. Although the precise incidence of this adverse effect is not well known, it is probably quite rare. Physicians should consider checking serum amylase and lipase levels for patients who develop nausea, vomiting and epigastric pain when taking doxycycline and/or ornidazole. It is important to consider doxycycline and/or ornidazole as a possible etiology for acute pancreatitis in patients presenting with gastrointestinal symptoms, even after a few days of doxycycline and/or ornidazole exposure. If doxycycline and/or ornidazole are suspected as the causative agent, they should then be discontinued and rechallenge should be avoided.
The authors have no potential conflict of interest