- (2009) Volume 10, Issue 4
Muhammad Wasif Saif
Yale University School of Medicine. New Haven, CT, USA
Despite attempted curative resection of localized pancreatic adenocarcinoma, most patients succumb a recurrence and die of their disease. The Gastrointestinal Tumor Study Group, European Organization for Research and Treatment of Cancer, and European Study Group for Pancreatic Cancer trials have suggested the benefit of adjuvant therapy. However, the relatively few randomized trials available have not established a definite standard of care due to study limitations. Although these trials, and the recently published Charité Onkologie (CONKO)-001 trial, have shown a definite advantage of adjuvant chemotherapy, the most effective chemotherapy and the role of radiation therapy remain unclear. This review will discuss the data available from reported trials of adjuvant therapy in pancreatic cancer, especially the results of the ESPAC-3 study presented at the annual meeting of ASCO 2009, and consider future directions for clinical trials.
capecitabine; Chemotherapy, Adjuvant; erlotinib; Fluorouracil; gemcitabine; Pancreatic Neoplasms; Radiotherapy
CONKO: Charité Onkologie; EORTC: European Organization of Research and Treatment of Cancer; ESPAC: European Study Group for Pancreatic Cancer; GITSG: Gastrointestinal Tumor Study Group; LV: leucovorin; RTOG: Radiation Therapy Oncology Group
Despite attempted curative resection of localized pancreatic adenocarcinoma, most patients succumb a recurrence and die of their disease. The Gastrointestinal Tumor Study Group, European Organization for Research and Treatment of Cancer, and European Study Group for Pancreatic Cancer trials have suggested the benefit of adjuvant therapy. However, the relatively few randomized trials available have not established a definite standard of care due to study limitations. Although these trials, and the recently published Charité Onkologie (CONKO)-001 trial, have shown a definite advantage of adjuvant chemotherapy, the most effective chemotherapy and the role of radiation therapy remain unclear. This review will discuss the data available from reported trials of adjuvant therapy in pancreatic cancer, especially the results of the ESPAC-3 study presented at the annual meeting of ASCO 2009, and consider future directions for clinical trials.

The high risk of local and systemic disease recurrence, as well as overall poor prognosis, laid down the rationale for adjuvant therapy after resection of pancreatic adenocarcinoma [1, 2].
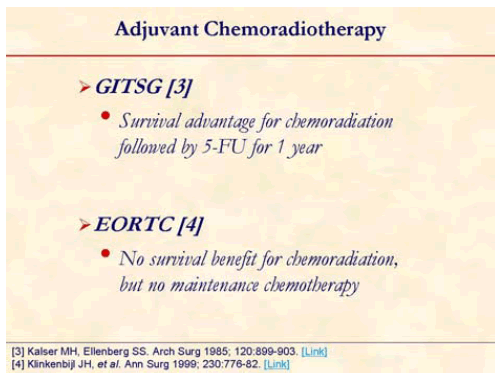
The GITSG trial was the first prospective randomized trial suggesting survival advantage with postoperative chemo-radiotherapy using bolus 5-FU (median survival: 20 months vs. 11 months; 5-year survival: 18% vs. 8%) [3]. However, this study was criticized for poor patient accrual, early termination, and small patient numbers, and some maintained that the radiotherapy dose was suboptimal (some authors advocate 50 Gy as a total effective dose).
The European Organization of Research and Treatment of Cancer (EORTC) compared 5-FU with concurrent radiotherapy using a split course with observation only in patients with resected pancreatic and periampullary cancer [4]. The subgroup analysis looking only at pancreatic cancer patients showed a trend toward benefit in median survival (17.1 months vs. 12.6 months; P=0.099) [4]. This study too was criticized for suboptimal dose of radiotherapy and split courses.
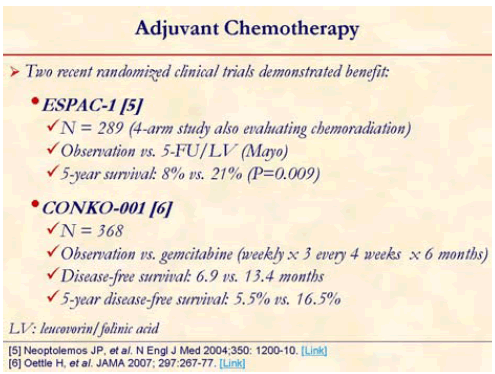
ESPAC-1 trial sparked a new debate over the role of radiotherapy in the adjuvant therapy of pancreatic cancer [5]. ESPAC-1 trial was 2x2 factorial designed study comparing adjuvant concurrent chemoradiotherapy (bolus 5-FU/split course radiotherapy), chemotherapy alone (5-FU/leucovorin), chemoradiotherapy followed by chemotherapy, and observation. Chemotherapy only arm had statistically significant benefit over observation arm in median survival (20.1 months vs. 15.5 months; P=0.009). However, chemoradiotherapy arm showed worse median survival compared with patients who did not receive chemo-radiotherapy (15.9 months vs. 17.9 months; P=0.05) [5]. Major criticism was made on this study for possible selection bias as both patients and clinicians were allowed to select which trial to enter, a concern of suboptimal radiotherapy, and for allowing the final radiotherapy dose to be left to the judgment of the treating physicians.
CONKO-001 study randomized 368 patients with resected pancreatic cancer to gemcitabine or observation for 6 months [6]. This trial showed statistically significant disease free survival benefit (13.4 months vs. 6.9 months; P<0.001) of gemcitabine over observation. Gemcitabine rendered a trend toward overall benefit (22.1 months vs. 20.2 months; P=0.06). This benefit of chemotherapy was consistent with the result from ESPAC-1 trial.
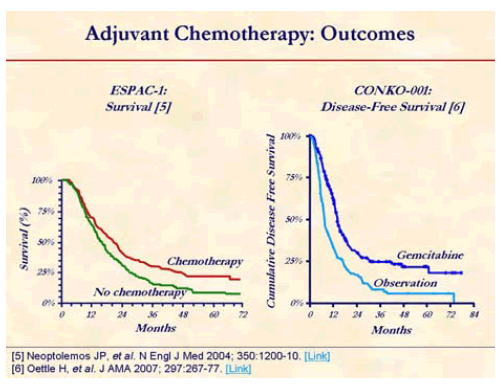
This benefit of chemotherapy in CONKO-001 study was consistent with the result from ESPAC-1 trial which showed benefit of 5-FU/leucovorin over no adjuvant therapy in pancreatic cancer patients (median survival of 19.7 months vs. 14.0 months) who had complete resection [5, 6].
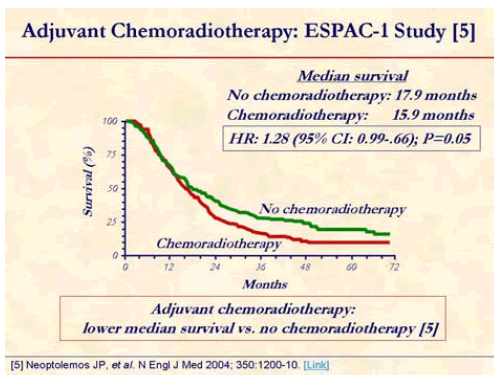
Chemoradiotherapy arm showed worse median survival compared with patients who did not receive chemoradiotherapy (15.9 months vs. 17.9 months; P=0.05) [5].
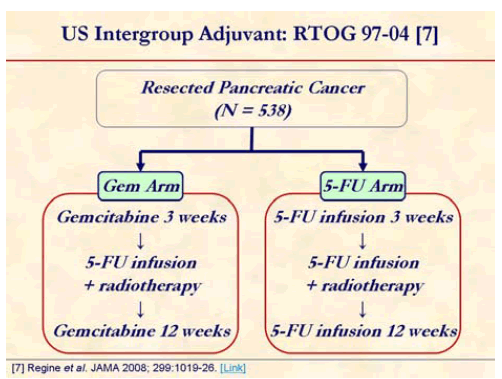
RTOG-Intergroup #97-04 was the 1st American cooperative group study since the GITSG trials. The study had no observation alone arm. The chemoradiation is common to both arms. The study asks the question of which is the better chemotherapy agent with respect to: 1) overall survival; 2) locoregional disease control; 3) distant disease control and failure patterns. CA 19-9 will be evaluated prospectively to predict outcome [7].
Chemotherapy with either fluorouracil (continuous infusion of 250 mg/m2 per day; n=230) or gemcitabine (30-minute infusion of 1,000 mg/m2 once per week; n=221) for 3 weeks prior to chemoradiation therapy and for 12 weeks after chemoradiation therapy. Chemoradiation with a continuous infusion of fluorouracil (250 mg/m2 per day) was the same for all patients (50.4 Gy).
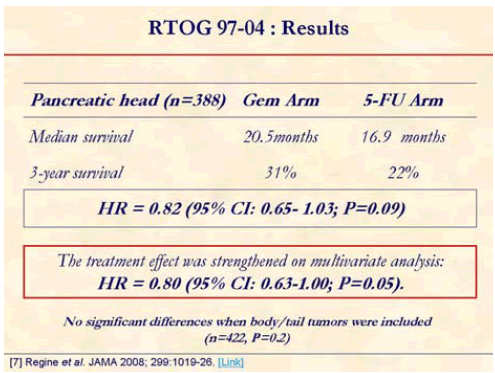
The addition of gemcitabine to adjuvant fluorouracilbased chemoradiation was associated with a survival benefit for patients with resected pancreatic cancer, although this improvement was not statistically significant. Forty-two percent crossed over to gemcitabine [7].
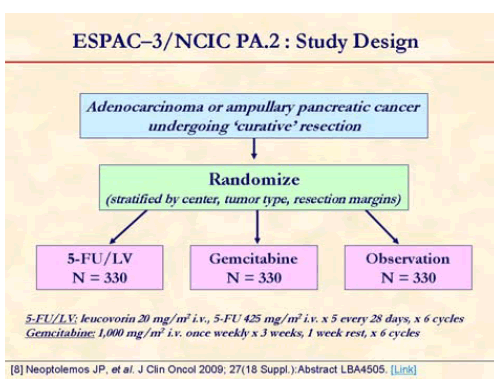
Neoptolemos et al. presented the results of ESPAC-3 study at the annual meeting of ASCO in June 2009 in Orlando, FL, USA. This is a multicenter, international, open-label, randomized, controlled, phase III trial that aimed at comparing of adjuvant 5- fluorouracil/leucovorin (5-FU/LV) versus gemcitabine in patients with resected pancreatic ductal adenocarcinoma [8]. Patients with an R0/R1 resection for pancreatic ductal adenocarcinoma were randomized (stratified for resection margin status and country) starting before 8 weeks of surgery to receive either 5- FU/LV (leucovorin, 20 mg/m2, i.v. bolus injection followed by 5-FU, 425 mg/m2, i.v. bolus injection given 1-5 days every 28 days) or gemcitabine (1,000 mg/m2 i.v. infusion at days 1, 8, and 15 every 4 weeks) for 6 months.
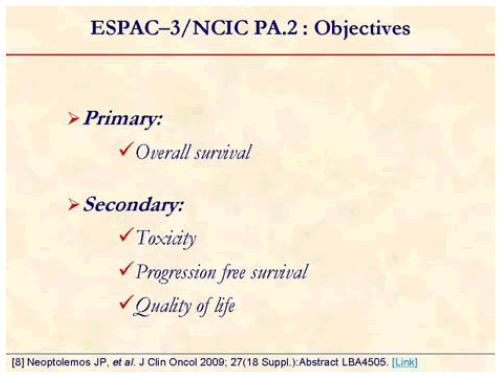
The primary outcome measure was overall survival; the secondary measures were toxicity, progression free survival and quality of life [8].
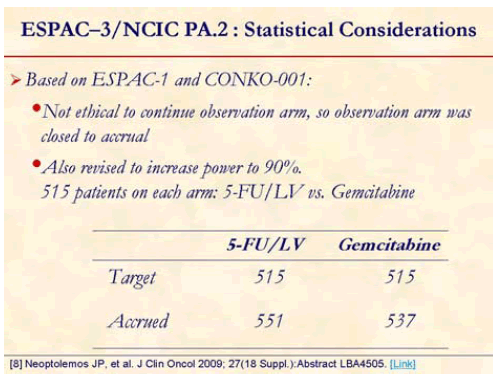
ESPSC-3 was revised to (v2) close the observation arm [8].
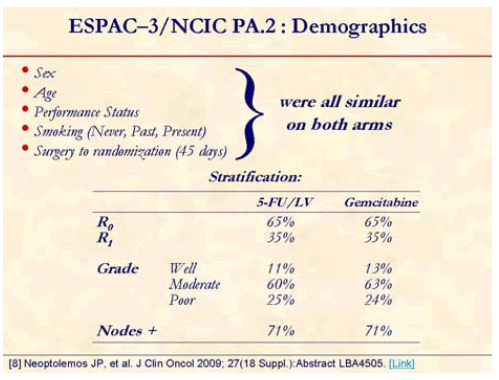
One-thousand and 88 patients from 16 countries were randomized from July 2000 to January 2007 (5-FU/LV, n=551; gemcitabine, n=537). Median age was 63 years and 55% were men. Thirty-five percent were R1 resections and approximately over 70% were node positive [8].
The study confirmed the role of adjuvant chemotherapy. However, overall survival was similar on both arms, hence showing that gemcitabine is not superior to 5-FU in adjuvant setting. Median overall survival was 23.0 months (95% CI: 21.1-25.0 months) with 5-FU/FA and it was 23.6 months (95%CI: 21.4- 26.4 months) with gemcitabine. There was no significant difference in the effect of treatment across subgroups according to R status (P=0.56). The study also confirmed the role of prognostic factors (whatever adjuvant chemotherapy): grade, stage, nodal status, resection status [8].
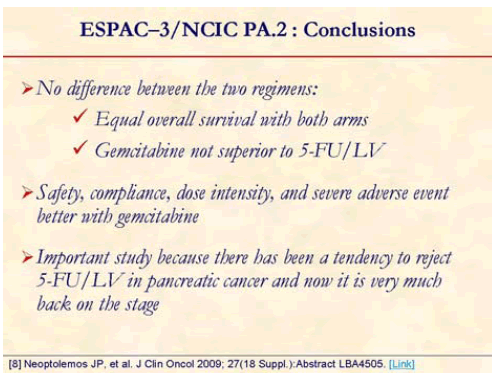
The study showed that gemcitabine is not superior to 5- FU in adjuvant setting; however, safety and dose intensity favors gemcitabine. This study is very important because there has been a tendency to reject 5-FU in pancreatic cancer and now it is very much back on the stage [8].
The author has no potential conflicts of interest