Mini Review - (2022) Volume 7, Issue 4
Aluminium Neurotoxicity and Neuroprotection.
Ljiljana Martac*,
Jelena Podgorac,
Branka Petkovic and
Gordana Stojadinovic
Department of Neurophysiology, University of Belgrade, Serbia
*Correspondence:
Ljiljana Martac, Department of Neurophysiology, University of Belgrade,
Serbia,
Email:
Received: 12-Jul-2022, Manuscript No. ipjhmct-22-13860;
Editor assigned: 14-Jul-2022, Pre QC No. ipjhmct-22-13860 (PQ);
Reviewed: 28-Jul-2022, QC No. ipjhmct-22-13860;
Revised: 02-Aug-2022, Manuscript No. ipjhmct-22-13860 (R);
Published:
09-Aug-2022, DOI: 10.21767/2473-6457.22.7.4.11
Abstract
Aluminium is considered to be the most widely distributed metal in nature and industry and is extensively used in
products and processes associated with human activity. Contamination may occur by air, water, food, additives, medicaments,
vaccines, cosmetics, agrochemicals, etc. Aluminium is recognized as a highly neurotoxic element in animals
and humans connected with several diseases such as Alzheimer’s and Parkinson’s disease, neurodegenerative
motor disorders, encephalopathy, dementia, amyotrophic lateral sclerosis, multiple sclerosis, and autism. There are
many animal models in rats developed to investigate aluminium neurotoxicity. Nevertheless, molecular mechanisms
of its action are not yet resolved, and mechanisms of damage and safety concentrations are still much discussed.
The brain is the most susceptible system to damages provoked by aluminium exposure, such as oxidative stress,
iron dyshomeostasis, changes in neurotransmission, immunologic alteration and pro-inflammation, genotoxicity,
transformation and peptide denaturation, changes in enzyme activity, membrane perturbation, apoptosis, necrosis,
and dysplasia. A novel investigation of aluminium neurotoxicity includes the assessment of neuroprotection and the
identification of new substances as potential drugs.
Keywords
Aluminium; Brain; Cognitive and Motor Diseases.
Introduction
Aluminium (Al) is a lightweight silvery white metal of main Group
13 (IIIa, or boron group) of the periodic table. It is the most widespread
metal on Earth, making up more than 8% of the Earth’s
core mass, and also the third most common chemical element on
our planet after oxygen and silicon. Al accumulates into the body
through different routes, induces various neurotoxic effects, represents
a risk factor in many neurodegenerative diseases, and its
side effects may be mitigated by the use of some neuroprotective
agents (Figure 1).
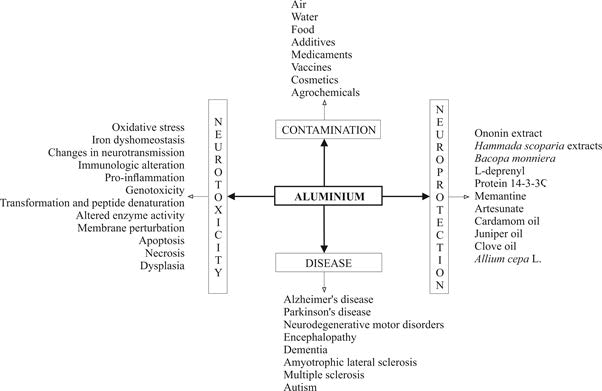
Figure 1: Schematic representation of the Al contamination routes, Al neurotoxicity and neuro protection, and Al-related diseases.
Aluminium as a Toxic Element
Al is widely spread in nature as a trivalent ion (Al+3) in silicates,
oxides, and hydroxides, as well as in combination with chlorine,
sulfur, fluorine, or organic matter [1]. Intake of Al is by air, water,
food, additives, medicaments, vaccines, cosmetics, agrochemicals,
etc. It is in extensive human use in different products such
as Al chloride, Al hydroxide, Al nitrate, Al phosphate, Al sulfate, and Al silicate [2]. Al ion has no physiological part in metabolic
processes, but it accumulates in mammalian tissue and has toxic
and pathologic effects [3,4]. Absorbed through the skin, intestinal
and alveolar mucosa, Al enters the brain across the blood
brain barrier (BBB), the choroid plexuses, and the nasal cavity
and remains for a long time since its removal from the brain tissue
is slow [5-8]. The distribution of Al in the brain is about 1% of
the total body, in all regions with maximum accumulation in the
hippocampus [9-11].
Al has multiple effects on cellular homeostasis and exhibits a
pro-oxidant activity that results in oxidative stress, free radical
attack, and oxidation of proteins and lipids [7]. It also induces
pro-inflammatory and pro-apoptotic gene expression, and affects
enzyme activity, and adenosine triphosphate (ATP) energy
metabolism. [12-14].
Aluminium Induced Oxidative Stress, Apoptosis
and Inflammation
Oxidative stress and changes in energy metabolism and mitochondrial function are the first events that make the brain sensitive
to Al accumulation [15]. In Al-loaded cells is observed loss of
christie, chromatin condensation, and decreased number of mitochondria
[16]. Oxidative stress is associated with a significant
reduction in antioxidant enzyme activity: superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and glutathione-
S-transferase with enhanced activity of nitric oxide (NO)
levels in some parts of the brain [17]. Induction of apoptosis in
cells exposed to Al includes several mechanisms: mitochondrial
pathway, p53, Bax, and caspase activation [18-20].
Different Al contractions affect the apoptosis of astrocytes (induce
or block selectively the process). On the one side, there is a
change in cell cycle distribution and increased intracellular Ca2+
at a dose of 400 μM of Al, whereas the dose of 200 μM of Al
blocks the apoptotic process [21]. As a result of these activities,
oxidative injury occurs and triggers neuroinflammation and microglial
activation. At the place of oxidative injury, the expression
of pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and MIP-1a is
increased; while the expression of brain derived neurotrophic
factor is significantly reduced [22,23]. Microglia activates the
secretion of IL-1β and other substances typical for microgliosis
inducing memory and learning dysfunction through modulation
of prostaglandin E2 synthase-prostaglandin E2-prostaglandins receptors
(PGES-PGE2-EPs) signaling pathway [24,25]. Particularly,
oxidative stress dependent glial activation in the rat brain is also
observed after Al exposure [26].
Al induces endoplasmic reticulum stress, which alters Ca2+ homeostasis
[27]. Given the role of the endoplasmic reticulum in
Ca2+ handling, altered intracellular Ca2+ levels may be indicative
of its dysfunction [28]. Due to toxic events, synaptic plasticity and
transmission are reduced, as well as neurotrophin production.
Synaptic dysfunction is a consequence of the inhibition of synaptic
Na+/K+-ATPase activity and a decrease in nerve growth factor
and brain derived neurotrophic factor expression [29,30]. Axonal
transport and perikaryal aggregation are altered in the cytoskeleton,
which may lead to neurofibrillary degeneration [31].
Discussion
Aluminium-Induced Changes in Neurotansmission
The central nervous system is the most susceptible to Al toxicity
and absorption and accumulation of Al in different brain regions
have an impact on glutamatergic, GABAergic, serotonergic,
cholinergic, and dopaminergic neurotransmission [32,33]. It
has been shown that Al reduces N-methyl-D-aspartate (NMDA)
and alpha-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid
(AMPA) expression, glutamate receptors playing an important
role in learning and memory, and fast excitatory glutamatergic
neurotransmission, respectively [34-36]. It also increases glutamate
levels in the cerebrum, thalamus, hippocampus, and
cerebellum, while as a response to the increased glutamatergic
transmission, GABAergic inhibitory effect is stimulated [37].
Under conditions of Al exposure, the cholinergic system shows
a marked reduction in acetylcholinesterase (AChE) activity, muscarinic
receptor binding, and nicotinic acetylcholine receptors
activity and gene expression [38,39]. Finally, Al exposure significantly
inhibits dopaminergic transmission and affects serotonin
levels differently due to a complex network of serotonin receptor
subtypes [40,41].
Animal Models of Aluminium Neurotoxicity
Al is a neurotoxic element implicated in several neurochemical,
neuropathological, electrophysiological, and behavioral changes associated with cognitive impairment [42]. For investigation of Al
neurotoxicity, different animal models are used. The most representative
is the animal model in rats, which mimics some diseases
occurring due to Al exposure. The neurotoxic properties of Al
exposure depend on several factors including dose, duration and
route of exposure, chemical forms, metabolism, accumulation,
detoxification and distribution, and elimination. Al application is
followed by differences in tissue distribution between the blood
and the target site [43]. Parenteral administration of Al exhibits
higher toxicity than oral application [44]. Also, young pups are
more sensitive than adults to Al exposure [45]. Cognitive decline
can be behaviorally tested on sensory, motor, and learning abilities.
The behavioral tests in animals include visual, motor, sensorimotor,
gross motor, and fine motor performances and reflexes,
coordination and locomotion [46].
According to our previous studies, spectral and fractal analysis
of the electrical activity in the brain has proven to be a reliable
tool for qualitative and quantitative assessment of changes in
the central nervous system in an animal model of intoxication
with Al [45,47-50]. So, a higher presence of power spectra in the
delta range of parietal electrocortical activity, a lower presence
in the theta range, and increased values of the parameter DT as
the ratio of delta to theta range were observed in pups indirectly
exposed to Al (whose mothers were drinking a 0.5% water solution
of Al chloride during the gestation and lactation periods),
compared to controls [45]. In adult male rats, the average fractal
dimension of electrocortical activity in chronically Al-treated animals
was lower than in the control rats, at cerebral but not at
cerebellar level [45-47].
Aluminium Related Diseases
Acute exposure to Al can cause clinical neurotoxicity. Encephalopathy
occurs among workers in the Al industry, and the main
symptoms are cognitive deficit, in-coordination, tremor, and
spinocerebellar degeneration [51]. Al in vaccines can cause neuroinflammation,
cell loss, and memory deficit [52]. Sporadic cases
include a seizure disorder, ataxia, and dysarthria. Al levels in
the brain are increasing with age, which may lead to neurodegenerative
diseases [53]. Alzheimer’s and Parkinson’s disease are
the most common Al-related diseases. Alzheimer’s disease develops
in the areas where the Al concentration in drinking water
is higher, and the main symptoms are dementia, development
of amyloid plaques consisting of aggregated β-amyloid proteins
and neurofibrillary tangles consisting of aggregated tau proteins,
production of reactive oxygen species, reactive microglia, and
the production of pro-inflammatory cytokines and macrophage
activity [54]. Al exposure may induce the disorder in dopamine
related brain regions, mostly the striatum, and together with inflammation
and microglial activation lead to Parkinson’s disease
[55,56]. In rat spinal cord, Al treatment causes severe motor
neuron damage resembling amyotrophic lateral sclerosis [57].
Acting as a pro-oxidant or as adjuvant inducing autoimmunity, [7]
Al may be involved in myelin loss and axonal degeneration that
occurs in multiple sclerosis [58]. The presence of Al in inflammatory
cells in the meninges, vasculature, grey, and white matter
could implicate Al in the etiology of autism [59].
Neuroprotection against Aluminium Toxicity
A novel investigation is focused on the mechanisms of neuro protection and many substances have been tested on animal models
of diseases but potential drugs have not yet been found. Shortly
we report some of these studies [60]. It is known that Alzheimer’s
disease in the initial phase is characterized by changes in
mood and behavior, aggression, confusion, avoidance of social
connections, and memory loss, while oxidative stress, inflammation,
and apoptosis are dysregulated and implicated in the progression
of the disease [61]. Ononin extract in an animal model
of Alzheimer’s disease suppresses oxidative stress and neuroinflammation,
activates apoptosis, prevents Al accumulation in the
brain, and stimulates cognitive impairment [62]. Hammada scoparia
extracts can be used for the treatment of Al neurotoxicity
due to the inhibitory effect on AChE activity and recovery from
oxidative damage induced by free radicals [63]. Bacopa monniera
and L-deprenyl also show neuroprotective efficiency through the
prevention of Al-induced oxidative damage and oxidative stress
[64]. Protein 14-3-3ζ combing with tau can prevent over phosphorylation
of tau, so it has a neuroprotective effect, which has
been experimentally proved in the hippocampus of rats [65]. Another
study in rats examined the protective effects of memantine
and artesunate in Al chloride-induced toxicity [66]. Both substances
reduce the cerebral level of TNF-α and IL-1β. Memantine,
as an NMDA receptor antagonist, reduces AChE activity, while
artesunate improves cognition, has an anti-inflammatory effect,
and attenuates oxidative stress. Cardamom oil has been reported
to have AChE inhibitory, antioxidant, and anti-anxiety effects
[67]. Also, similar activity has juniper oil and clove oil [68]. Allium
cepa L. has neuroprotective effects on Al chloride-induced neurotoxicity
by improving muscle coordination and memory deficits
[69]. It reduces oxidative stress, AChE activity, and Al deposition
in the brain.
Conclusion
This work is focused on the consequences of contamination with
Al, as a highly neurotoxic element, on the central nervous system
and provides insight into the main damages caused by Al in the
brain, cognitive and motor diseases associated with exposure to
Al, and possible mechanisms of neuroprotective action of various
agents in conditions of Al intoxication. It summarizes the current
state of knowledge on the topic and represents a basis for future
research and predictions of Al neurotoxicity and neuroprotection.
Authors Contributions
All authors participated in the writing of the manuscript; LM conceptualized
and wrote the original draft of the manuscript, JP, BP,
and GS reviewed and edited the manuscript.
Acknowledgements
None.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect
to the authorship and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support
for the authorship and/or publication of this article: This work
was supported by the Ministry of Education, Science, and Technological
Development of the Republic of Serbia [contract number
451-03-68/2022-14/200007].
REFERENCES
- Martin RB (1992) Aluminium speciation in biology. Ciba Found Symp 169:525.
[Crossref] [Google Scholar] [PubMed]
- Lewis RJ (2001) Hawley,'s condensed chemical dictionary, 14th ed. Wiley-Interscience: New Jersey, USA pp. 3946.
- Exley C, House ER (2011) Aluminium in the human brain. Monatshefte fur Chemie 142(4):35763.
[Crossref] [Google Scholar]
- Bogdanović M, Janeva AB, Bulat P (2008) Histopathological changes in rat liver after a single high dose of aluminium. Arh Hig Rada Toksikol 59(2):97101.
[Crossref] [Google Scholar] [PubMed]
- Flarend R, Bin T, Elmore D, Hem SL (2001) A preliminary study of the dermal absorption of aluminium from antiperspirants using aluminium-26. Food Chem Toxicol 39(2):1638.
[Crossref] [Google Scholar] [PubMed]
- Cunat L, Lanhers MC, Joyeux M, Burnel D (2000) Bioavailability and intestinal absorption of aluminum in rats: Effects of aluminum compounds and some dietary constituents. Biol Trace Elem Res 76(1):3155.
[Crossref] [Google Scholar] [PubMed]
- Exley C (2013) Human exposure to aluminium. Environ Sci Process Impacts 15(10):180716.
[Crossref] [Google Scholar] [PubMed]
- Wang L (2018) Entry and deposit of aluminum in the brain. Adv Exp Med Biol 1091:3951.
[Crossref] [Google Scholar] [PubMed]
- Yokel RA, McNamara PJ (2001) Aluminium toxicokinetics: An updated minireview. Pharmacol Toxicol 88(4):15967.
[Crossref] [Google Scholar] [PubMed]
- Julka D, Vasishta RK, Gill KD (1996) Distribution of aluminum in different brain regions and body organs of rat. Biol Trace Elem Res 52(2):18192.
[Crossref] [Google Scholar] [PubMed]
- Sánchez-Iglesias S, Soto-Otero R, Iglesias-González J, Barciela-Alonso MC, Bermejo-Barrera P, et al. (2007) Analysis of brain regional distribution of aluminium in rats via oral and intraperitoneal administration. J Trace Elem Med Biol 21(Suppl 1):314.
[Crossref] [Google Scholar] [PubMed]
- Lukiw WJ, Percy ME, Kruck TP (2005) Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. J Inorg Biochem 99(9):18958.
[Crossref] [Google Scholar] [PubMed]
- Sushma NJ, Sivalah U, Suraj NJ, Rao KJ (2007) Aluminium acetate: Role in oxidative metabolism of albino mice. Int Zool Res 3(1):4852.
[Crossref] [Google Scholar] [ResearchGate]
- Kawahara M, Konoha K, Nagata T, Sadakane Y (2007) Aluminum and human health: Its intake, bioavailability and neurotoxicity. Biomed Res Trace Elements 18(3):21120.
[Crossref] [Google Scholar]
- Kumar V, Gill KD (2014) Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: A review. Neurotoxicology 41:15466.
[Crossref] [Google Scholar] [PubMed]
- Sharma DR, Sunkaria A, Wani WY, Sharma RK, Kandimalla RJ, et al. (2013) Aluminium induced oxidative stress results in decreased mitochondrial biogenesis via modulation of PGC-1α expression. Toxicol Appl Pharmacol 273(2):36580.
[Crossref] [Google Scholar] [PubMed]
- Skalny AV, Aschner M, Jiang Y, Gluhcheva YG, Tizabi Y, et al. (2021) Molecular mechanisms of aluminum neurotoxicity: Update on adverse effects and therapeutic strategies. Adv Neurotoxicol 5:134.
[Crossref] [Google Scholar] [ScienceDirect]
- Savory J, Herman MM, Ghribi O (2003) Intracellular mechanisms underlying aluminum-induced apoptosis in rabbit brain. J Inorg Biochem 97(1):1514.
[Crossref] [Google Scholar] [PubMed]
- Johnson VJ, Kim SH, Sharma RP (2005) Aluminum-maltolate induces apoptosis and necrosis in neuro-2a cells: Potential role for p53 signaling. Toxicol Sci 83(2):32939.
[Crossref] [Google Scholar] [PubMed]
- Mesole SB, Alfred OO, Yusuf UA, Lukubi L, Ndhlovu D (2020) Apoptotic inducement of neuronal cells by aluminium chloride and the neuroprotective effect of eugenol in Wistar rats. Oxid Med Cell Longev 2020:8425643.
[Crossref] [Google Scholar] [PubMed]
- Guo GW, Liang YX (2001) Aluminum-induced apoptosis in cultured astrocytes and its effect on calcium homeostasis. Brain Res 888(2):2216.
[Crossref] [Google Scholar] [PubMed]
- Cao Z, Yang X, Zhang H, Wang H, Huang W, et al. (2016) Aluminum chloride induces neuroinflammation, loss of neuronal dendritic spine and cognition impairment in developing rat. Chemosphere 151:28995.
[Crossref] [Google Scholar] [PubMed]
- Prema A, Justin Thenmozhi A, Manivasagam T, Mohamed Essa M, Guillemin GJ (2017) Fenugreek seed powder attenuated aluminum chloride-induced tau pathology, oxidative stress, and inflammation in a rat model of Alzheimer's disease. J Alzheimers Dis 60(s1):S20920.
[Crossref] [Google Scholar] [PubMed]
- Blaylock RL (2012) Aluminum induced immunoexcitotoxicity in neurodevelopmental and neurodegenerative disorders. Curr Inorg Chem 2(1):4653.
[Crossref] [Google Scholar] [ResearchGate]
- Guo Y, Lei W, Wang J, Hu X, Wei Y, et al. (2016) Misoprostol reverse hippocampal neuron cyclooxygenase-2 downstream signaling imbalance in aluminum-overload rats. Curr Alzheimer Res 13(9):100616.
[Crossref] [Google Scholar] [PubMed]
- Akinrinade ID, Memudu AE, Ogundele OM, Ajetunmobi OI (2015) Interplay of glia activation and oxidative stress formation in fluoride and aluminium exposure. Pathophysiology 22(1):3948.
[Crossref] [Google Scholar] [PubMed]
- Aremu DA, Ezomo OF, Meshitsuka S (2011) Gene expression in primary cultured astrocytes affected by aluminum: Alteration of chaperons involved in protein folding. Environ Health Prev Med 16(1):1624.
[Crossref] [Google Scholar] [PubMed]
- Rizvi SHM, Parveen A, Verma AK, Ahmad I, Arshad M, et al. (2014) Aluminium induced endoplasmic reticulum stress mediated cell death in SH-SY5Y neuroblastoma cell line is independent of p53. PLoS One 9(5):e98409.
[Crossref] [Google Scholar] [PubMed]
- Silva VS, Gonçalves PP (2003) The inhibitory effect of aluminium on the (Na+/K+)ATPase activity of rat brain cortex synaptosomes. J Inorg Biochem 97(1):14350.
[Crossref] [Google Scholar] [PubMed]
- Johnson VJ, Sharma RP (2003) Aluminum disrupts the pro-inflammatory cytokine/neurotrophin balance in primary brain rotation-mediated aggregate cultures: Possible role in neurodegeneration. Neurotoxicology24(2):2618.
[Crossref] [Google Scholar] [PubMed]
- Kushkuley J, Metkar S, Chan WK, Lee S, Shea TB (2010) Aluminum induces neurofilament aggregation by stabilizing cross-bridging of phosphorylated c-terminal sidearms. Brain Res 1322:11823.
[Crossref] [Google Scholar] [PubMed]
- Exley C, Mold MJ (2019) Aluminium in human brain tissue: How much is too much? J Biol Inorg Chem 24(8):127982.
[Crossref] [Google Scholar] [PubMed]
- Gonçalves PP, Silva VS. (2007) Does neurotransmission impairment accompanies aluminium neurotoxicity? J Inorg Biochem 101(9):1291338.
[Crossref] [Google Scholar] [PubMed]
- Platt B, Haas H, Büsselberg D (1994) Aluminium reduces glutamate-activated currents of rat hippocampal neurones. Neuroreport 5(17):232932.
[Crossref] [Google Scholar] [PubMed]
- Newcomer JW, Farber NB, Olney JW (2000) NMDA receptor function, memory, and brain aging. Dialogues Clin Neurosci 2(3):21932.
[Crossref] [Google Scholar] [PubMed]
- Watson JF, Ho H, Greger IH (2017) Synaptic transmission and plasticity require AMPA receptor anchoring via its N-terminal domain. Elife 6:e23024.
[Crossref] [Google Scholar] [PubMed]
- Nayak P, Chatterjee AK (2001) Effects of aluminium exposure on brain glutamate and GABA systems: An experimental study in rats. Food Chem Toxicol 39(12):12859.
[Crossref] [Google Scholar] [PubMed]
- Julka D, Sandhir R, Gill KD (1995) Altered cholinergic metabolism in rat CNS following aluminum exposure: Implications on learning performance. J Neurochem 65(5):215764.
[Crossref] [Google Scholar] [PubMed]
- Farhat SM, Mahboob A, Ahmed T (2021) Oral exposure to aluminum leads to reduced nicotinic acetylcholine receptor gene expression, severe neurodegeneration and impaired hippocampus dependent learning in mice. Drug Chem Toxicol 44(3):3108.
[Crossref] [Google Scholar] [PubMed]
- Laabbar W, Elgot A, Elhiba O, Gamrani H (2019) Curcumin prevents the midbrain dopaminergic innervations and locomotor performance deficiencies resulting from chronic aluminum exposure in rat. J Chem Neuroanat 100:101654.
[Crossref] [Google Scholar] [PubMed]
- Kumar S (2002) Aluminium-induced changes in the rat brain serotonin system. Food Chem Toxicol 40(12):187580.
[Crossref] [Google Scholar] [PubMed]
- Ćulić M, Martać L, Grbić G, Kesić S, Spasić S, et al. (2007) Aluminum toxicity in rat brain: Electrophysiological, histological and behavioral evidence. In: Gantchev N (ed.) from basic motor control to functional recovery V. Sofia: Sofia Publ House, pp. 22430.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Ning ZH, Tai HW, Long S, Qin WC, et al. (2015) Relationship between lethal toxicity in oral administration and injection to mice: Effect of exposure routes. Regul Toxicol Pharmacol 71(2):20512.
[Crossref] [Google Scholar] [PubMed]
- Ogasawara Y, Sakamoto T, Ishii K, Takahashi H, Tanabe S (2002) Effects of the administration routes and chemical forms of aluminum on aluminum accumulation in rat brain. Biol Trace Elem Res 86(3):26978.
[Crossref] [Google Scholar] [PubMed]
- Martać L, Grbić G, Keković G, Podgorac J, Ćulić M, et al. (2010a) Spectral changes of brain activity in rat offspring exposed to aluminum during gestation and lactation. Arch Biol Sci 62(1):913.
[Crossref] [Google Scholar] [ResearchGate]
- Ingber SZ, Pohl HR (2016) Windows of sensitivity to toxic chemicals in the motor effects development. Regul Toxicol Pharmacol 74:93104.
[Crossref] [Google Scholar] [PubMed]
- Keković G, Ćulić M, Martać L, Stojadinović G, Čapo I, et al. (2010) Fractal dimension values of cerebral and cerebellar activity in rats loaded with aluminium. Med Biol Eng Comput 48(7):6719.
[Crossref] [Google Scholar] [PubMed]
- Martać L, Podgorac J, Sekulić S (2010b) Evaluation of the neurotoxical effect of aluminum on the Wistar Rat. Arch Biol Sci 62(3):5858.
[Crossref] [Google Scholar] [ResearchGate]
- Martać L, Podgorac J, Sekulić S, Čapo I (2014) Animal model of neurodegeneration and stress cause by aluminium toxicity. American Journal of BioScience 2(2):2831.
[Google Scholar]
- Martać L, Podgorac J, Petković B, Sekulić S, Čapo I (2015) Spectral and fractal analysis of ECoG in animal model of aluminium intoxication. J Biotech Res 1(5):215.
[Google Scholar]
- Polizzi S, Pira E, Ferrara M, Bugiani M, Papaleo A, et al. (2002) Neurotoxic effects of aluminium among foundry workers and Alzheimer's disease. Neurotoxicology 23(6):76174.
[Crossref] [Google Scholar] [PubMed]
- Petrik MS, Wong MC, Tabata RC, Garry RF, Shaw CA (2007) Aluminum adjuvant linked to Gulf War illness induces motor neuron death in mice. Neuromolecular Med 9(1):83100.
[Crossref] [Google Scholar] [PubMed]
- Jansson ET (2001) Aluminum exposure and Alzheimer's disease. J Alzheimers Dis 3(6):5419.
[Crossref] [Google Scholar] [PubMed]
- Bondy SC (2010) The neurotoxicity of environmental aluminum is still an issue. Neurotoxicol 31(5):57581.
[Crossref] [Google Scholar] [PubMed]
- Shirabe T, Irie K, Uchida M (2002) Autopsy case of aluminum encephalopathy. Neuropathol 22(3):20610.
[Crossref] [Google Scholar] [PubMed]
- Nagatsu T, Sawada M (2005) Inflammatory process in Parkinson's disease: Role for cytokines. Curr Pharm Des 11(8):9991016.
[Crossref] [Google Scholar] [PubMed]
- Tanridag T, Coskun T, Hürdag C, Arbak S, Aktan S, et al. (1999) Motor neuron degeneration due to aluminium deposition in the spinal cord: A light microscopical study. Acta Histochem 101(2):193201.
[Crossref] [Google Scholar] [PubMed]
- Exley C, Swarbrick L, Gherardi RK, Authier FJ (2009) A role for the body burden of aluminium in vaccine-associated macrophagic myofasciitis and chronic fatigue syndrome. Med Hypotheses 72(2):1359.
[Crossref] [Google Scholar] [PubMed]
- Mold M, Umar D, King A, Exley C (2018) Aluminium in brain tissue in autism. J Trace Elem Med Biol 46:7682.
[Crossref] [Google Scholar] [PubMed]
- Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, et al. (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396(10248):41346.
[Crossref] [Google Scholar] [PubMed]
- Gan L, Johnson JA (2014) Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys Acta 1842(8):120818.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Zhang M, Ahmed M, Surapaneni KM, Veeraraghavan VP, et al. (2021) Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer's disease in rats. Saudi J Biol Sci 28(8):42329.
[Crossref] [Google Scholar] [PubMed]
- Taïr K, Kharoubi O, Taïr OA, Hellal N, Benyettou I, et al. (2016) Aluminium induced acute neurotoxicity in rats: Treatment with aqueous extract of Arthrophytum (Hammada scoparia). J Acute Dis 5(6):47082.
[Crossref] [Google Scholar] [ResearchGate]
- Jyoti A, Sethi P, Sharma D (2007) Bacopa monniera prevents from aluminium neurotoxicity in the cerebral cortex of rat brain. J Ethnopharmacol 111(1):5662.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Cheng D, Jiang W, Ma Y (2018) Mechanisms underlying aluminum neurotoxicity related to 14-3-3ζ protein. Toxicol Sci 163(1):4556.
[Crossref] [Google Scholar] [PubMed]
- Shata A, Elkashef W, Hamouda M, Eissa H (2020) Effect of artesunate vs memantine in aluminum chloride induced model of neurotoxicity in rats. Adv Alzheimer's Dis 9:119.
[Crossref] [Google Scholar]
- Auti ST, Kulkarni YA (2019) Neuroprotective Effect of cardamom oil against aluminum induced neurotoxicity in rats. Front Neurol 10:399.
[Crossref] [Google Scholar] [ResearchGate]
- Dohi S, Terasaki M, Makino M. (2009) Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J Agric Food Chem 57(10):43138.
[Crossref] [Google Scholar] [PubMed]
- Singh T, Goel RK (2015) Neuroprotective effect of Allium cepa L. in aluminium chloride induced neurotoxicity. Neurotoxicology 49:17.
[Crossref] [Google Scholar] [PubMed]
Citation: Ljiljana Martac, Jelena Podgorac, Branka Petkovic, Gordana Stojadinovic (2022) Aluminium Neurotoxicity and Neuroprotection. J Heavy Met Toxicity Dis. 7:11.
Copyright: © Ljiljana M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.