- (2007) Volume 8, Issue 6
Zeng-Gang Pan, Bo Wang
Department of Pathology, Creighton University Medical Center. Omaha, NE, USA
Received July 9th, 2007 - Accepted September 6th, 2007
Context Anaplastic carcinoma of the pancreas is a rare undifferentiated variant of ductal adenocarcinoma, which commonly displays sarcomatoid spindle-cell and pleomorphic growth patterns. Anaplastic carcinoma of the pancreas associated with mucinous cystic neoplasm has rarely been reported. Case report Here we report a unique case of an anaplastic carcinoma of the pancreas in association with a mucinous cystadenocarcinoma in a 70-year-old woman. The anaplastic component of this tumor is predominantly composed of spindle cells and highly pleomorphic cells that mimic a spindle cell sarcoma. The spindle neoplastic cells have strong expression of vimentin and mucin 1 and focal strong positivity of CK7 and CK20. Scattered osteoclast-like giant cells are admixed with the spindle cells with positivity for CD68 but not epithelial or other mesenchymal markers. Focal squamoid differentiation is present. Adjacent to the solid anaplastic tumor is a classic mucinous cystadenocarcinoma, which has strong reactivity to mucin 1, CA 19-9, epithelial membrane antigen (EMA), CK19, CK8/18, carcinoembryonic antigen and CK7. The pericystic tissue and the septa consist of an ovarian-type stroma that is strongly positive for CD10. Focal areas with pancreatic intraepithelial neoplasia IB (PanIN-IB) changes are seen in the adjacent normal pancreatic tissue. Conclusion The anaplastic carcinoma of the pancreas is of epithelial origin with various microscopic features, and the scattered osteoclast-like giant cells in the tumor are reactive cells of histiocytic origin.
Carcinoma; Cystadenocarcinoma, Mucinous; Pancreas
Anaplastic carcinoma of the pancreas is a rare pancreatic tumor of epithelial origin, frequently showing various morphologies that include pleomorphic epithelial cells and relatively mononuclear spindle cells. Other rare growth patterns have been reported, including rhabdoid and squamous patterns. Despite of many morphology changes, the neoplastic cells usually have reactivity to epithelial markers and vimentin, indicating an epithelial origin with dedifferentiation. Diagnosis of this type of tumor may be challenging due to lack of the glandular structures or other features that indicate a direction of differentiation. However, it is very important to recognize this distinct entity because of the highly aggressive nature of this type of tumor. In our report, this 70-year-old woman had a pleomorphic anaplastic carcinoma of the pancreas in adjacent to a mucinous cystadenocarcinoma and a PanINIB. A brief review of the literature is included.
A 70-year-old Caucasian female presented with a two-month history of a funny taste in the mouth, anemia, loss of appetite and a weight loss of about 18 pounds. Past medical history was significant for hypertension and appendectomy. During this hospitalization, an extensive workup showed a 6.0 cm right breast mass, and an invasive ductal adenocarcinoma was reported following a biopsy. A further CT scan of the abdomen revealed a large complex cystic and solid mass measuring 10.4x8.3x6.6 cm in the body and tail of the pancreas (Figure 1a). The patient then underwent an exploratory laparotomy with extensive distal pancreatectomy and splenectomy. During the surgery, a local advanced tumor was resected without evidence of systemic metastasis. The patient’s condition remains controlled and stable after four months following the surgery.
Figure 1. a. CT scan of the abdomen reveals a large complex cystic and solid mass in the body and tail of the pancreas. b. Gross specimen demonstrates a well-circumscribed tumor with a solid component and a polycystic component in the body and tail of the pancreas. Microscopically, the solid component in the tumor consists of a variety of features: c. mononuclear spindle cells; d. highly pleomorphic epithelial cells; e. scattered multinucleated osteoclast-like giant cells with reaction to CD68 as indicated in the insert; f. squamoid differentiation.
Grossly, the specimen consists of a resected spleen and a distal portion of the pancreas. The spleen is unremarkable and free of tumor invasion. The pancreatic specimen measures 14x12x9 cm, and the surface of the specimen is smooth but distorted by multiple large bulging nodules. Sectioning through the pancreatic mass reveals a well-circumscribed tumor with a solid component and a multicystic component (Figure 1b). The solid area of the tumor is pale tan and yellow measuring 8.0x4.5 cm. The cysts contain brown-tan cloudy gelatinous material with the largest cyst measuring 4.0x3.5 cm.
Microscopically, the tumor has a relatively well-demarcated pushing border with focal invasion into the adjacent normal pancreatic tissue. The tumor is restricted in the capsule without evidence of infiltration to the major pancreatic vessels or distant metastasis. The solid area of the tumor consists of mononuclear spindle cells and highly pleomorphic epithelial cells with marked mitotic index (Figure 1c, 1d). The tumor cells are large with indistinct cell boarders and moderate to abundant eosinophilic cytoplasm. The nuclei are large, spindle, oval or round with marked pleomorphism, hyperchromasia and one or two conspicuous nucleoli (Figure 1d). Many bizarre giant tumor cells are seen, and hemorrhage and necrosis are prominent in the tumor. Occasional multinucleated osteoclast-like giant cells are admixed with the spindle tumor cells in focal areas (Figure 1e), and these osteoclast-like giant cells are reactive to CD68 (Figure 1e insert). Nerve invasion (Figure 2b and 2c) and squamoid differentiation (Figure 1f) are identified. There is no glandular differentiation in the solid area of the tumor.
The cysts in the tumor are lined with mucinous epithelium with focal papillary projections (Figure 3c). The lining epithelium cells appear highly pleomorphic and atypical, and a mucinous cystadenocarcinoma is reported. A section of the tumor also demonstrates a transition of the mucinous cystadenocarcinoma (Figure 3a, upper left and lower right; Figure 3b, lower right) and the anaplastic tumor (Figure 3a, center; Figure 3b, upper left). The anaplastic component at this point is negative for majority of the markers that are detected in the lining epithelium, including CK7 and CK8/18 (Figure 4b, lower right; 4c, upper left). The peri-cystic tissue and the septa contain focal calcifications and a distinctive ovarian-type stroma, which is composed of dense spindle cells with sparse cytoplasm and elongated wavy nuclei.
Figure 3. a. This figure shows the transition of the mucinous cystadenocarcinoma (upper left and lower right) and the anaplastic tumor (center). b. Higher power view of the lining epithelium of the cyst (lower right) and the anaplastic tumor (upper left). c. Additional sections of the cyst reveals the lining mucinous epithelium with high grade dysplasia. d. The PanIN-IB in the normal pancreatic tissue.
A pancreatic intraepithelial neoplasia (PanINIB, Figure 3d) is present in the duct of adjacent normal pancreatic tissue. This lesion consists of a papillary epithelium that is lined by single layer of uniform columnar cells with minimal atypia. The lining epithelial cells contain abundant supranuclear mucin and have basally located, round to oval uniform nuclei that are oriented perpendicular to the basement membranes.
Immunostains of the anaplastic carcinoma demonstrate that the spindle and highly pleomorphic tumor cells have strong positivity of vimentin (Figure 2d) and mucin 1 (MUC-1) (Figure 2c), focal strong positivity of CK7 (Figure 2a) and CK20 (Figure 2b), and weak positivity of beta-catenin and p53. The tumor cells are negative for cytokeratin AE1/AE3 (CK AE1/AE3), CK5/6, CK8/18, desmin and CD10. The scattered osteoclast- like giant cells are strongly reactive to CD68 (Figure 1e, insert) but negative for epithelial markers. The lining epithelium of the mucinous cystadenocarcinoma shows strong positivity for CA 19-9 (Figure 4a), CK7 (Figure 4b), CK8/18 (Figure 4c), CK19 (Figure 4d) and MUC-1 (Figure 4e). The stroma of the cystic septa is strongly positive for CD10 (Figure 4f).
Ultrastructurally, the neoplastic cells have variable sizes and shapes with prominent nucleoli but no predominance of any specific cytoplasmic organelles. Cytogenetic analysis did not reveal any detectable numerical or structural chromosomal anomaly.
Anaplastic carcinoma of the pancreas is a rare aggressive pancreatic tumor and accounts for 2-7% of all pancreatic cancers with a male predominance [1, 2]. A number of terms have been used to describe this type of tumor, including pleomorphic carcinoma, sarcomatoid carcinoma, anaplastic and undifferentiated carcinoma. Anaplastic carcinomas of the pancreas are more common in older men with an age peak in the seventh-ninth decades of life. The clinical symptoms include loss of weight, fatigue, loss of appetite, abdominal pain, nausea, vomiting and diarrhea [1, 2, 3]. Our patient had many of these classic symptoms.
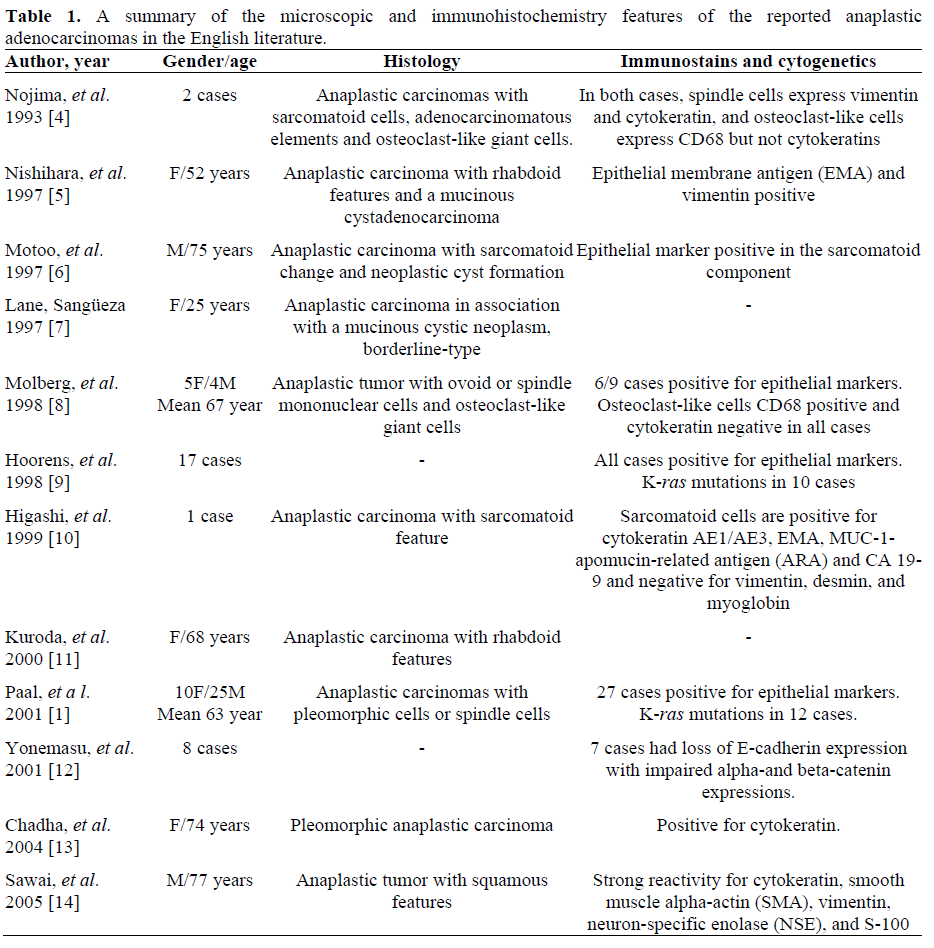
This tumor may occur in the head, body and tail of the pancreas. Three major histological subtypes have been described, spindle cell carcinoma, pleomorphic carcinoma and round cell carcinoma (Table 1). Anaplastic carcinomas with other very rare features have been reported, including two cases with rhabdoid features [5, 11] and one with squamous features [15]. Despite of various morphology changes, the neoplastic cells usually have reactivity to epithelial markers and vimentin, indicating an epithelial origin with dedifferentiation. Anaplastic carcinomas of pancreas in association with mucinous cystic neoplasm were rarely reported although the mucinous cystic neoplasm may be a precursor lesion. Only two cases were reported in the English literatures, one cystadenocarcinoma [5] and the other one borderline type [7].
Interestingly, many of the anaplastic carcinomas contain osteoclast-like giant cells. There has been some disagreement regarding to the origin of these osteoclast-like giant cells in pancreatic tumor, and the proposed origins of the osteoclast-like giant cells include neoplastic epithelial cells, neoplastic mesenchymal cells and reactive mesenchymal cells. Many studies have shown that these giant cells highly express CD68 and lysozyme without reactivity to epithelial markers [4, 8], and they have similar morphological features to the osteoclast-like giant cells in the bone. There findings suggest a histiocytic origin of these giant cells and they may be reactive cells in the pancreatic tumor. However, when the pancreatic tumor has prominent osteoclast-like giant cells, it may belong to another distinct rare entity of pancreatic carcinoma, named undifferentiated carcinoma with osteoclast-like giant cells.
The tumor in our case has a solid component with a predominance of spindle neoplastic cells with admixture of highly pleomorphic epithelial cells. Immunohistochemically, the spindle cells have strong reactivity to MUC-1 and vimentin with focal strong positivity for CK7 and CK20, indicating an epithelial origin of these spindle cells. The tumor also has focal squamoid differentiation. Interestingly, a large mucinous cystadenocarcinoma is present in our case. The mucinous cystadenocarcinoma highly expresses MUC-1, CA 19- 9, epithelial membrane antigen (EMA), CK19, CK8/18, carcinoembryonic antigen (CEA) and CK7, and the cystic septa consist of a CD10 positive ovarian-type stroma. This type of mucinous cystadenocarcinomas almost always occurs in the female. In our case, the scattered osteoclast-like giant cells have strong reaction to CD68 but not epithelial markers, which further suggests that they are reactive cells of histiocytic origin. Focal PanIN-IB is also noted in the adjacent normal pancreatic tissue.
Most anaplastic carcinomas of the pancreas harbor activating point mutations in the codon 12 of K-ras oncogene. In pancreatic tumors with ductal and anaplastic components, both components reveal identical point mutation of the K-ras oncogene, indicating that the anaplastic carcinoma may arise from the ductal carcinoma. It has been postulated that mutations of the K-ras oncogene occur at early stages of PanIN progression, and inactivation of the p16 tumor suppressor gene occurs at intermediate stages, and the inactivation of the p53, SMAD4 (DPC4), and BRCA 2 tumor suppressor genes occur at late stages of malignant transformation [15]. PanIN is a common lesion in normal elderly adults, and the PanIN-IB in our case may be an incidental finding.
Anaplastic pancreatic carcinoma is an aggressive neoplasm with a worse prognosis than poorly differentiated ductal adenocarcinoma of the pancreas. The 3-year survival rate is lower than 3%, with an expectation of 10 to 20 months. Compared with poorly differentiated pancreatic ductal adenocarcinoma, anaplastic carcinoma has a loss or impaired expression of surface adhesion molecules, including E-cadherin, alpha- and beta-catenin [12], which may explain some of the aggressive natures of this tumor. Interestingly, the tumor in our case is pathologically and clinically not as aggressive as those described in the literature in spite of its large size and typical anaplastic features. The tumor is locally restricted without evidence of extensive invasion or distant metastasis, and the patient is stable after the surgery. Possible explanation is that this tumor may not acquire enough cellular and molecular alterations for its invasion and distant metastasis.
The authors have no potential conflicts of interest