Keywords
Screen-printed electrodes,Antimony film, Copper, Dolomite, Limestone, Fly ash extract
Introduction
Copper belongs to trace elements essential for biological systems, but it is toxic at higher concentrations [1]. The natural levels of copper in surface water, soil and rock vary significantly, but some carbonate rocks are rich in it. For example, the mineral malachite (copper carbonate hydroxide) occurs together with limestone. If either of these materials, after grinding, is used as a soil conditioner or dietary supplement (dolomite only), providing calcium and magnesium for people or animal food, there is a risk of copper contamination. The concentration of copper in nature may also be influenced by anthropogenic emissions, including those from industrial waste. The passage of water or an aqueous solution through porous material (slag or ash heaps) along with the diffusion of contaminants through material may cause the release of toxic substances to the environment. The precise analytical control of copper levels is therefore very important.
To determine low concentrations of copper in minerals, mainly spectrometric methods are used. These methods include electro thermal atomic absorption spectrometry (ET-AAS), inductively coupled plasma optical emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS). However, the determination of copper traces in dolomites, limestone, and fly ash extracts by means of ET-AAS or of ICP-OES may be problematic due to matrix effects caused by the presence of high concentrations of Ca and Mg in the tested samples.
Samples containing a high concentration of sulfates may also be problematic; such samples include water extracts of sulfate-rich fly ash produced during the combustion of carbon in a fluidized bed, where lime is added to fuel to prevent the emission of sulfur oxides. The lime reacts with SO2 to form sulfates, which become part of the fly ash.
In contrast, anodic stripping (ASV) and adsorptive stripping voltammetry, which require relatively inexpensive instrumentation, are sufficiently sensitive for copper quantification in highly concentrated solutions. The hanging mercury drop electrode (HMDE) or the mercury film electrode have been commonly used for the sensitive detection of the above element by means of the ASV technique [2,3]. Unfortunately, the ASV curves of copper in the vast majority of the supporting electrolytes are partly overlapped by the current from the anodic dissolution of mercury, which makes correct measurements of the Cu response at low concentrations difficult. Moreover, due to restrictions in the manipulation and disposal of mercury and its salts in laboratories in some countries, new electrode materials for the preparation of voltammetric sensors have been proposed and tested. Especially bismuth (BiFE) [4] and, subsequently, antimony (SbFE) film electrodes [5,6], both introduced to the analytical practice at the beginning of the present century, have found wide application for the ASV detection of some metal ions (e.g., Pb, Cd, Zn, In, Tl) and organic compounds [7-11]. Unfortunately, the standard potential of copper is more positive than those of Bi or Sb and the anodic Cu oxidation wave is located outside the accessible potential windows of bismuth and antimony film electrodes.
In spite of the above, Wang et al. demonstrated that, using the BiFE working electrode, a split Cu anodic stripping voltammetric signal was observed at the potential more positive than that of the Bi dissolution peak [12]. However, the applicability of the BiFE for the detection of Cu traces in real samples is limited due to the competition of the electrodeposited copper and the bismuth for surface sites [12,13] and the partial overlapping of the Bi and Cu oxidation waves [14-16]. The addition of gallium or hydrogen peroxide alleviated these issues [17-19]. The application of the antimony film electrodes (SbFE) for the detection of Cu appeared to be more attractive because of the lower sensitivity of the Sb anodic wave in comparison to that of Bi and consequently, its lower influence on the Cu response. Therefore, mainly SbFEs deposited in situ on the glassy carbon [20,21] and carbon paste [22,23] supports have been proposed for the determination of Cu, although the tin film electrode (SnFE) has also been used for this purpose [24]. It was evidenced that the most sensitive Cu signals at the SbFE deposited in situ on the glassy carbon or carbon paste supports were observed in such supporting electrolytes as hydrochloric acid [20,22,23] or potassium tartrate [21]. Due to the insensitive response of Sb in 0.01 M hydrochloric acid, it was possible to determine a relatively low Cu concentration, although the Cu and Sb anodic peaks were almost identical [23]. On the other hand, when potassium tartrate was used as the supporting electrolyte, the differences in peak potentials of Sb-Cu pair amounted to 160 mV, providing good separation of both signals [21].
Glassy carbon and carbon paste have been commonly used as supports for the preparation of metal film electrodes (BiFE, SBFE, SnFE) plated in situ or ex situ [7-11]. Recently, screenprinted electrodes (SPE) have been attracting increasing interest in stripping voltammetry as an alternative substrate for metal film formation because of their many advantages, such as mass production, low cost, small size, lower susceptibility to oxygen interferences, and the possibility of disposal after a single use [25- 30]. SPEs work with portable, battery-operated electrochemical analysers, making it possible to test the level of toxic metals on site (e.g., stockpiles of raw materials at production sites, in areas contaminated by leakage from industrial installations, ash or slag heaps). SPEs modified with metal layers are a modern type of sensors, and thanks to their advantages they further broaden the scope of applications of electroanalytical methods. The protocols for the determination of Pb and Cd using anodic stripping voltammetry (ASV) employing SPEs have been extensively investigated. However, those describing the determination of Cu are less widespread because of unfavorably positive oxidation potential. Mercury film and gold-sputtered screen-printed electrodes have first been exploited in a voltammetric study of Cu contents [31,32]. So far, only the recently published paper by Arrino et al. has described the application of a modified SPE with an antimony film for the ASV determination of copper [33]. The authors proposed acetate buffer as a suitable supporting electrolyte for the sensitive determination of Cu, Cd and Pb at the SbFE deposited in situ. The attempts to apply SbF-SPEs have so far been focused on the detection of Cu in certified groundwater [34].
The aim of the presented study was to evaluate the possibility of utilizing anodic stripping voltammetry for the determination of copper in carbonate minerals and water extracts of fly ash using the modified screen-printed graphite electrode. To simplify the course of experiment the antimony film was deposited in situ after spiking the examined solution with Sb(III) ions. To obtain the optimal response of the sensing system, it was necessary to test the influence of chemical and instrumental factors on the copper signal and to also evaluate the influence of main constituents of the samples’ matrix.
Experimental
Reagents
All solutions were prepared using deionized water with resistivity of 18 MΩ (Millipore Simplicity UV, USA). 1 M acetate buffer of pH 4.5 was prepared by mixing appropriate amount of CH3COONH4 with CH3COOH solution (Suprapur, Merck). 1 M potassium nitrate was prepared by dissolving appropriate amount of reagent in water. Standard solutions containing Sb(III) or Cu(II) at a concentration of 1 g L-1 were obtained from Merck and diluted as required.
Apparatus
All measurements were performed using an Autolab potentiostat (EcoChemie, The Netherlands) connected to the PC with GPES 4.9 software. The screen-printed electrodes (4 mm in diameter; carbon DRP C110) were provided by Dropsens, Spain. The working electrode used in all experiments was antimony film plated in situ at a screen-printed carbon electrode (SPCE). A coil of platinum wire served as the counter electrode, and an Ag/AgCl electrode (3 M KCl) (Metrohm, Switzerland) was used as the reference electrode. Photolysis was performed using a UV digestion system with a 150 W lamp (Mineral, Poland).
Measurement procedure
The electrochemical investigations were carried out by means of differential pulse anodic stripping voltammetry. The antimony film was deposited in situ at the surface of the screen-printed carbon electrode (SPE). The film-forming Sb(III) was added directly to the examined solution. An aliquot of the analyzed sample was pipetted into the electrochemical cell. After that, the following reagents were added: 0.1 ml of 1 M acetate buffer, 0.025 ml of a 0.1 g L-1 Sb(III) solution, and 0.1 ml of 1 M KNO3. Finally, deionized water was added to the total volume of 10 ml and dissolved oxygen was removed via 7 minutes of argon purging. Afterwards, the potential of 0.5 V was applied for 30 s to clean the surface of the working electrode. Immediately after cleaning, the potential was changed to -1.0 V and maintained for 60 s to deposit the antimony film and, at the same time, accumulate copper on the surface of the working electrode. During cleaning and accumulation, the solution was stirred using a magnetic bar. After 15 s of rest, a differential pulse voltammogram (DP-ASV) was recorded over the range of -0.4 V to 0.2 V. The modulation amplitude was 50 mV and the potential step was 4 mV.
Sample characteristics
Limestone and dolomite: The powdereddolomite and limestone samples (1 g of both) were digested in 10 ml of 65% HNO3 (Suprapur, Merck) on a hot plate for 30 minutes, then filtered, transferred to a 100 ml flask and UV-irradiated for 2 h. To speed up the photolysis process, 10 μl of 30% H2O2 was added to each 10 ml of the sample.
Fly ash extract: The fly ash samples originated from thermal power stations in Poland equipped with fluidized bed lignite-fired boiler (fly ash 1) or conventional bituminous coal-fired boiler (fly ash 2). The extraction procedure was performed according to EN 12457-2:2002. During this procedure, the samples were leached with distilled water (room temperature) at a sample-to-solvent ratio of 1:10. The mixture was sealed in polypropylene extraction bottles and placed in a rotary agitator (36 rpm, bottles placed at an angle of 45 degrees to the axis of rotation) for 24 h. After mixing, the samples were filtered, and the pH and conductivity were measured. The fraction of the extract used for voltammetric determination was acidified to pH 2 using concentrated HNO3 (Suprapur, Merck).
Results and Discussion
Evaluation of the performance of unmodified and antimony-plated screen-printed electrodes
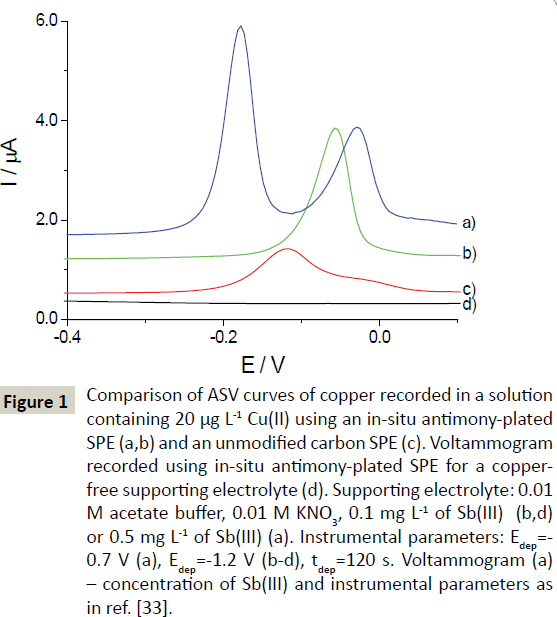
Unmodified screen-printed carbon electrodes can be used to record measurable ASV stripping signals of copper, but their shape and sensitivity are inadequate. The copper signal recorded in a solution containing 20 μg L-1 of Cu(II) is very extended – from -0.22 to 0.05 V – and relatively low (Figure 1). Such a distorted peak in pure acetate buffer makes unmodified SPE unsuitable for the analysis of real samples. Moreover, matrix components adversely affect the voltammetric signals. Adding 0.1 mgL-1 of Sb(III) ions to the examined solution made the determination of Cu(II) via ASV possible. During the electrolytic preconcentration of Cu(II) in the presence of Sb(III) ions at -1.0 V, Sb(III) ions were reduced to form the antimony film and, at the same time, copper accumulated on the surface of the SPE working electrode. The copper response recorded using the in-situ plated antimony SPE (SbF-SPE) was well-shaped, more narrow, and more sensitive (three times higher) than that obtained using the unmodified SPE (Figure 1). The potential of copper peak shifted to more positive values by 58 mV in comparison to that obtained using the unmodified SPE.
Figure 1: Comparison of ASV curves of copper recorded in a solution containing 20 μg L-1 Cu(II) using an in-situ antimony-plated SPE (a,b) and an unmodified carbon SPE (c). Voltammogram recorded using in-situ antimony-plated SPE for a copperfree supporting electrolyte (d). Supporting electrolyte: 0.01 M acetate buffer, 0.01 M KNO3, 0.1 mg L-1 of Sb(III) (b,d) or 0.5 mg L-1 of Sb(III) (a). Instrumental parameters: Edep=- 0.7 V (a), Edep=-1.2 V (b-d), tdep=120 s. Voltammogram (a) – concentration of Sb(III) and instrumental parameters as in ref. [33].
Optimization of chemical and instrumental variables
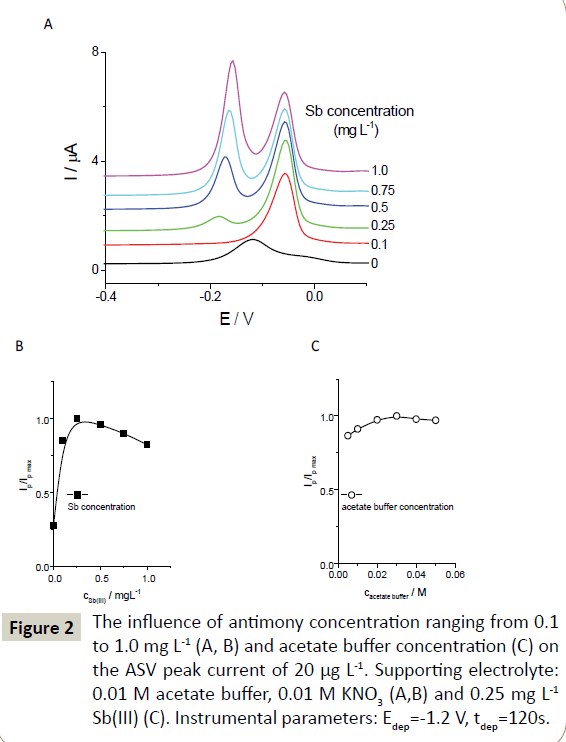
To select the optimal concentration of Sb(III) ions, the effect of its concentration on the copper signal was further investigated (Figure 2). At the Sb(III) ion concentration of 0.25 mg L-1, the copper peak was the highest and was 3.7 times higher than the copper signal recorded using the unmodified SPE. A small peak originating from antimony oxidation was also observed at a potential 0.1 V more negative than that of the copper peak. Increasing the Sb(III) concentration further resulted in a slight drop in the copper peak current (Figure 2A and 2B). In addition, the antimony signals recorded in solutions with a concentration higher than 0.25 mg L-1 partially distorted the copper signal.
Figure 2: The influence of antimony concentration ranging from 0.1 to 1.0 mg L-1 (A, B) and acetate buffer concentration (C) on the ASV peak current of 20 μg L-1. Supporting electrolyte: 0.01 M acetate buffer, 0.01 M KNO3 (A,B) and 0.25 mg L-1 Sb(III) (C). Instrumental parameters: Edep=-1.2 V, tdep=120s.
The Cu(II) signals recorded in solutions with acetate buffer concentrations ranging from 0.005 to 0.05 M did not differ to a considerable extent (the RSD of their peak currents was equal to 5.3%; Figure 2C). When increasing the concentration of acetate buffer within the investigated range, the potential of the Cu response shifted to more negative values, by 68 mV per 10 mM. Further experiments were therefore performed in 0.01 M acetate buffer containing 0.25 mg L-1 of Sb(III).
The pH of the supporting electrolyte has a profound effect on the preconcentration efficiency of metals and on the width of the potential window. The influence of the acetate buffer pH was investigated in the range 3.6 to 5.5 by adding the appropriate amount of NaOH. The highest, most narrow and most symmetrical copper signals were recorded in the acetate buffer with the pH of 4.5. In the case of solutions with a pH lower than 4.5, copper and antimony peaks overlapped; when the pH was higher than 4.5, copper peaks were lower and not symmetrical.
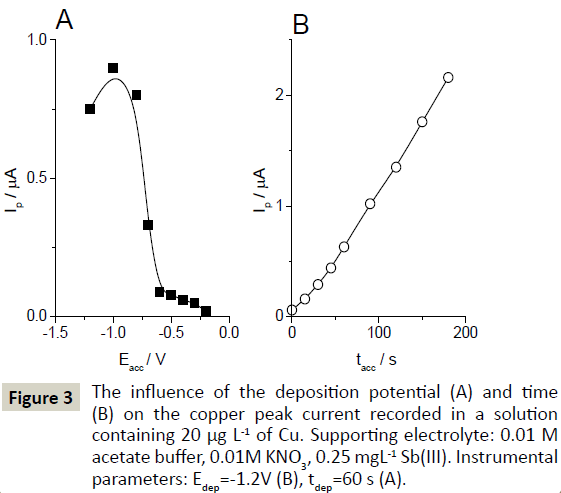
The DPV mode was found to be optimal for Cu(II) determination. The SWV and LSV techniques were not sufficiently sensitive to provide satisfactory signals of Cu(II). The influence of the deposition potential, one of the most important parameters affecting the efficiency of metal ion accumulation, was investigated in the range from -1.2 to -0.2 V. The copper signal increased from -1.2 V up to -1.0 V; less negative accumulation potentials caused the copper signal to decrease. The highest copper signal was obtained for the accumulation potential of -1.0 V, and this value was chosen for further investigations (Figure 2).
The results of tests performed at the antimony-plated SPE after accumulation at – 1.0 V in a solution of 20 μg L-1 of copper showed a linear increase in the copper signal in the accumulation time range of 0 - 180 s. For the majority of further experiments, the accumulation time of 60 s was used as a compromise between sensitivity and the duration of the procedure. In some experiments, accumulation was prolonged to 120 s to increase the sensitivity of the copper signal (Figure 3).
Figure 3: The influence of the deposition potential (A) and time (B) on the copper peak current recorded in a solution containing 20 μg L-1 of Cu. Supporting electrolyte: 0.01 M acetate buffer, 0.01M KNO3, 0.25 mgL-1 Sb(III). Instrumental parameters: Edep=-1.2V (B), tdep=60 s (A).
Analytical performance
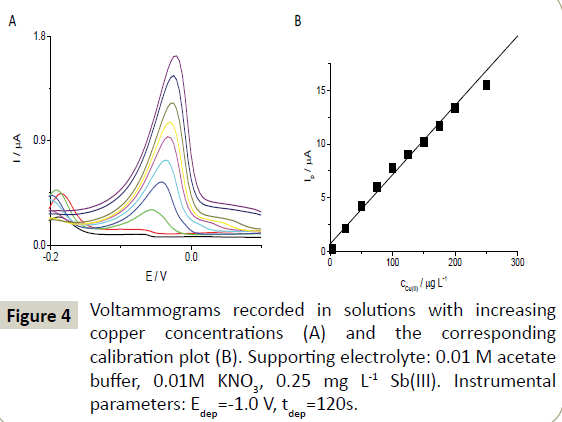
Figure 4 presents voltammograms recorded using the in-situ plated antimony SPE in the 0.01 M acetate buffer solution containing copper in concentrations ranging from 4 to 250 μg L-1. Under the optimized conditions, the examined SbF-SPE provided well-defined stripping curves with a peak current that depended linearly on Cu(II) concentration in a relatively wide range from 4 to 200 μg L-1 (y=(0.065 ± 0.002)x+(0.7 ± 0.3), where y and x denote the peak current (μA) and Cu(II) concentration (μg L-1), respectively; R2=0.99). The loss of linearity at concentrations higher than 200 μg L-1 was likely due to the partial saturation of the electrode surface. The detection limit for an accumulation time of 120 s estimated from 3 times the standard deviation for the lowest concentration of Cu(II) was equal to 0.2 μg L-1. The reproducibility estimated for 10 subsequent curves was satisfactory (RSD=5%) for voltammograms recorded in the solution containing 4 μg L-1 of Cu(II).
Figure 4: Voltammograms recorded in solutions with increasing copper concentrations (A) and the corresponding calibration plot (B). Supporting electrolyte: 0.01 M acetate buffer, 0.01M KNO3, 0.25 mg L-1 Sb(III). Instrumental parameters: Edep=-1.0 V, tdep=120s.
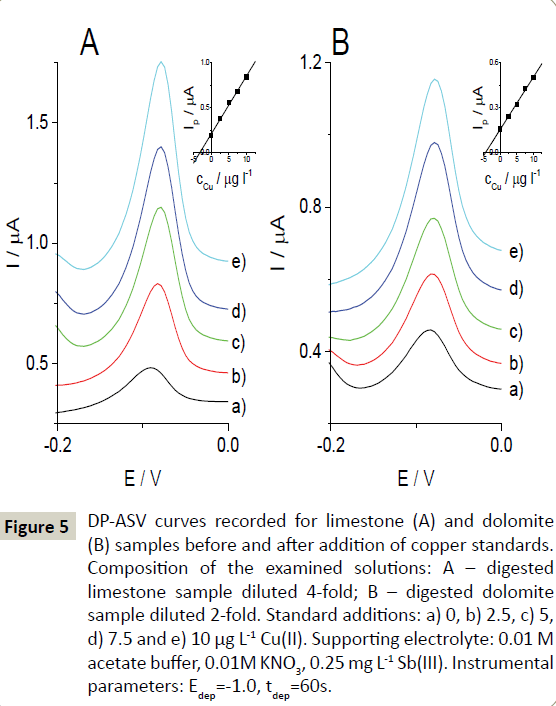
To validate the proposed protocol, Cu(II) was determined in the reference material (Reference Material for Measurement of Elements in Surface Waters SPS-SW2 Batch 129). Three subsamples were analyzed by applying the optimized chemical and instrumental parameters. The results of copper determination obtained using the in-situ SbF-SPE (96.6 ± 2.2 μg L-1) were in good agreement with the certified value of 100.0 ± 1 μg L-1( Figure 5).
Figure 5: DP-ASV curves recorded for limestone (A) and dolomite (B) samples before and after addition of copper standards. Composition of the examined solutions: A – digested limestone sample diluted 4-fold; B – digested dolomite sample diluted 2-fold. Standard additions: a) 0, b) 2.5, c) 5, d) 7.5 and e) 10 μg L-1 Cu(II). Supporting electrolyte: 0.01 M acetate buffer, 0.01M KNO3, 0.25 mg L-1 Sb(III). Instrumental parameters: Edep=-1.0, tdep=60s.
Interferences
All examined samples were rich in calcium ions; the fly ash extracts also contained a high amount of sulfate. The influence of Ca(II) on the copper signal was tested up to 0.025 M of Ca(II) and interferences were not observed. The sulfate ions did not affect the copper signal up to 0.2 M. Unlike Ca(II) and sulfate, chloride ions heavily influenced the copper signal. A discernible drop in the copper signal recorded in the solution containing 25 μg L-1 Cu(II) appeared for a NaCl concentration as low as 0.2 mM, and became progressively worse up to 0.011 M, at which point the copper peak retained only 15% of its initial value. Consequently, nitric acid was used to digest the solidlimestone and dolomite samples.
Application
The investigated in-situ SbF-SPE was applied for the determination of Cu(II) in the digested dolomite and limestone samples, and fly ash extracts. To determine the Cu(II) concentration, the standard additions procedure was used (Figure 5). Quantification was performed for three aliquots of each investigated sample. The results are presented in Table 1. In the case of fly ash extracts, the components of the solution matrix heavily influenced the Cu(II) response. The copper signals recorded in the pure supporting electrolyte were higher than those recorded in copper-spiked (20 μg L-1) samples of fly ash extracts. This signal suppression was observed in 20-fold diluted samples (45% and 21% drop in the copper signal for fly ash 1 and fly ash 2, respectively) and was more pronounced in the less diluted sample solutions. To assess if the suppressing effect was caused by the presence of organic matter originating from unburned carbon compounds, UVphotolysis was performed again for these samples. After 12 hours of photolysis, it was possible to record measureable copper peaks for the extract of fly ash 1. Unfortunately, the copper signal was still not present in the voltammograms recorded for the extract of fly ash 2. In this case, it was only possible to perform recovery studies after spiking the sample with 20 μg L-1 of Cu(II) (Table 1).
| Sample |
Concentration in solution(ASV-SPE)(µg L-1) |
Concentration in solution (ICP MS)(µg L-1) |
Contents*(mg kg-1) |
| Limestone |
13 ± 1 |
11.9 ± 0.9 |
1.3 |
| Dolomite |
8.8 ± 0.3 |
8.5 ± 0.2 |
0.88 |
| Fly ash extract 1 |
2.5 ± 0.2 |
2.3 ± 0.1 |
0.025** |
| Fly ash extract 2 |
Not detected
(Recovery 118%***) |
<1.5 |
- |
Table 1: Results of voltammetric determination of copper in mineral and industrial samples employing an in-situ SbF-SPE.
Conclusions
The introduction of Sb(III) ions into the acetate buffer for antimony film formation improved the sensitivity of the voltammetric determination of copper and the quality of the signals recorded using SPEs in terms of symmetry and peak width. Under the optimized conditions, the copper signal recorded using the SbF-SPE was 3.7 times higher than that observed when unmodified SPEs were used. The procedure offers one of the lowest limits of detection (0.2 μg L-1) and a linear response over a wide concentration range (4-200 μgL-1) when compared with other procedures employing SPEs. It was demonstrated that the proposed method is suitable for the determination of trace copper content in both inorganic natural (carbonate minerals) samples and materials produced by human activity (fly ash). The method is simple and fast, offers sufficient sensitivity, and utilizes relatively inexpensive electrochemical instrumentation. These features make it suitable for the future application in the on-site determination of trace levels of copper.
Acknowledgements
Financial support from the Polish National Science Centre (Project 2011/01/B/ST8/07794) is gratefully acknowledged.
References
- Kabata-Pendias A, Mukherjee AB (2007) Trace Elements from Soil to Human, Heidelberg, Berlin: Springer-Veilag.
- Wang J (2006) Analytical Electrochemistry. (3rd edn), Hoboken, John Wiley & Sons Ltd, New Jersey.
- Economou A, Fielden PR (2003) Mercury film electrodes: developments, trends and potentialities for electroanalysis. Analyst 128: 205-212.
- Wang J, Lu J, Hocevar SB, Farias PA, Ogorevc B, et al. (2000) Bismuth-coated carbon electrodes for anodic stripping voltammetry Anal Chem 72: 3218-3222.
- Pauliukaite R, Metelka R, Švancara I, Królicka A, Bobrowski A, et al. (2004) Screen Printed Carbon Electrodes Bulk-Modified with Bi2O3 or Sb2O3 for Trace Determination of Some Heavy Metals. Scientific Papers of the University of Pardubice, Series A 10: 47-58.
- Hocevar SB, Svancara I, Ogorevc B, Vytras K (2007) Antimony film electrode for electrochemical stripping analysis. Anal Chem 79: 8639-8643.
- Wang J (2005) Stripping Analysis at Bismuth Electrodes: A Review. Electroanal 17: 1341-1346.
- Economou A (2005) Bismuth-Film Electrodes: Recent Developments and Potentialities for Electroanalysis. TrAC – Trend. Anal. Chem 24: 334-340.
- Kokkinos C, Economou A (2008) Stripping Analysis at Bismuth-Based Electrodes. Curr Anal Chem 4: 183-190.
- Švancara I, Prior C, Hocevar SB, Wang J (2010) A Decade with Bismuth-Based Electrodes in Electroanalysis. Electroanal 22: 1405-1420.
- Lezi N, Economou A, Barek J (2013) Electrodes Modified with Bismuth, Antimony and Tin Precursor Compounds for Electrochemical Stripping Analysis of Trace Metals (a Short Review). Sensing in Electroanalysis 8: 9-20.
- Wang J, Lu J, Kirgöz UA, Hocevar SB, Ogorevc B, et al. (2001) Insight into The Anodic Stripping Voltammetric Behavior of Bismuth Film Electrodes. Anal Chim Acta 434: 29-34.
- Vytras K, Baldrianova L, Tesarova E, Švancara I, Bobrowski A, et al. (2005) Comments to Stripping Voltammetric Determination of Copper(II) at Bismuth-Modified Carbon Substrate Electrodes. Sensing in Electroanalysis 49-58.
- Baldo MA, Daniele S (2005) Anodic Stripping Voltammetry at Bismuth-Coated and Uncoated Carbon Microdisk Electrodes: Application to Trace Metals Analysis in Food Samples. Anal Lett 37: 995-1011.
- de Carvalho LM, do Nascimento PC, Koschinsky A, Bau M, Stefanello RF, et al. (2007) Simultaneous Determination of Cadmium, Lead, Copper, and Thallium in Highly Saline Samples by Anodic Stripping Voltammetry (ASV) Using Mercury-Film and Bismuth-Film Electrodes. Electroanal 19: 1719-1726.
- Cesarino I, Gouveia-Caridade C, Pauliukaite R, Cavalheiro ETG, Brett CMA, et al. (2010). Characterization and Application of Bismuth-Film Modified Graphite-Polyurethane Composite Electrodes. Electroanal 22: 1437-1445.
- Prior C, Lenehan CE, Walker GS (2006) Enhanced Resolution of Copper and Bismuth by Addition of Gallium in Anodic Stripping Voltammetry with the Bismuth Film Electrode. Electroanal 18: 2486-2489.
- Prior C, Lenehan CE, Walker GS (2007) Utilising gallium for enhanced electrochemical copper analysis at the bismuth film electrode. Anal Chim Acta 598: 65-73.
- Pacheco WF1, Miguel EM, Ramos GV, Cardoso CE, Farias PA, et al. (2008) Use of hydrogen peroxide to achieve interference-free stripping voltammetric determination of copper at the bismuth-film electrode. Anal Chim Acta 625: 22-27.
- Slavec M, Hocevar SB, Baldrianova L, Tesarova E, Svancara I, et al. (2010) Antimony Film Microelectrode for Anodic Stripping Measurement of Cadmium(II), Lead(II) and Copper(II). Electroanal 22: 1617-1622.
- Bobrowski A, Putek M, Zarebski J (2012) Antimony Film Electrode Prepared In Situ in Hydrogen Potassium Tartrate In Anodic Stripping Voltammetry Trace Detection of Cd(II), Pb(II) Zn(II), Tl(I), In(III) and Cu(II). Electroanal 24: 1071-1078.
- Ashra? AM, Vytras K (2012) New Procedure for Voltammetric Determination of Copper(II) Using Antimony Film-Coated Carbon Paste Electrodes. Electrochim Acta 73: 112-117.
- Ashra? AM, Husakova L, Vytras K (2012) Voltammetric Determination of Copper(II) Using Antimony Film Electrodes. Sensing in Electroanalysis 7: 189-199.
- Czop E, Economou A, Bobrowski A (2011) A Study of In Situ Plated Tin-Film Electrodes for The Determination of Trace Metals by Means of Square-Wave Anodic Stripping Voltammetry. Electrochim Acta 56: 2206-2216.
- Renedo OD, Alonso-Lomillo MA, Martínez MJ (2007) Recent developments in the field of screen-printed electrodes and their related applications. Talanta 73: 202-219.
- Metters JP, Kadara RO, Banks CE (2011) New directions in screen printed electroanalytical sensors: an overview of recent developments. Analyst 136: 1067-1076.
- Li M, Li YT, Li DW, Long YT (2012) Recent developments and applications of screen-printed electrodes in environmental assays--a review. Anal Chim Acta 734: 31-44.
- Serrano N, Alberich A, Diaz-Cruz JM, Arino C, Esteban M, et al. (2013) Coating Methods. Modi?ers and Applications of Bismuth Screen-Printed Electrodes. TrAC – Trend. Anal. Chem 46: 15-29.
- Hayat A, Marty JL (2014) Disposable screen printed electrochemical sensors: tools for environmental monitoring. Sensors (Basel) 14: 10432-10453.
- Niu X, Lan M, Zhao H, Chen C, Li Y, et al. (2013) Zhu X. Review: Electrochemical Stripping Analysis of Trace Heavy Metals Using Screen-Printed Electrodes. Anal Lett 46: 2479-2502.
- Laschi S, Palchetti I, Mascini M (2006) Gold-Based Screen-Printed Sensor for Detection of Trace Lead. Sensor. Actuat B 114: 460-465.
- Rueda-Holgado F, Bernalte E, Palomo-Marín MR, Calvo-Blázquez L, Cereceda-Balic F, et al. (2012) Miniaturized voltammetric stripping on screen printed gold electrodes for field determination of copper in atmospheric deposition. Talanta 101: 435-439.
- Sosa V, Barceló C, Serrano N, Ariño C, Díaz-Cruz JM, et al. (2015) Antimony film screen-printed carbon electrode for stripping analysis of Cd(II), Pb(II), and Cu(II) in natural samples. Anal Chim Acta 855: 34-40.
- Council decision of 19 December 2002 establishing criteria and procedures for the acceptance of waste at landfills pursuant to Article 16 of Annex II to Directive 999/31/EC (2003/33/EC).