- (2009) Volume 10, Issue 5
Jonathan M Buscaglia1,4, Christopher J DiMaio2, Michael J Pollack3, Eun Ji Shin1, Michael D Harris4, Robert Richards4, Amitabh Chak3, Sergey V Kantsevoy1, Sanjay B Jagannath1, Patrick I Okolo III1
1Division of Gastroenterology, Department of Medicine, Johns Hopkins University School of Medicine, Johns Hopkins Hospital. Baltimore, MD, USA.
2Division of Gastroenterology, Department of Medicine, Harvard Medical School, Massachusetts General Hospital. Boston, MA, USA.
3Division of Gastroenterology, Department of Medicine, Case Western Reserve University School of Medicine, University Hospitals of Cleveland. Cleveland, OH, USA.
4Division of Gastroenterology, Department of Medicine, State University of New York at Stony Brook, Stony Brook University Medical Center. Stony Brook, NY, USA
Received: 22 April 2009 Accepted: 15 July 2009
Context Pancreatic stents are frequently clogged at the time of removal. There is limited data regarding the factors associated with stent occlusion. Objectives To estimate the frequency of stent occlusion at the time of removal, to study the accuracy of endoscopic prediction of occlusion, and to determine the factors associated with clogged pancreatic stents. Setting Consecutive patients at 4 academic medical centers undergoing removal of a previously placed pancreatic stent were prospectively enrolled. Patients A total of 68 patients were enrolled between August 2007 and July 2008. Interventions Following removal, stent occlusion was immediately assessed by complete lack of water flow from the duodenal end and side holes of the stent. Main outcome measure Survival analysis was performed using a Kaplan-Meier and Cox Regression model. Results Indications for stent placement included chronic pancreatitis (n=23), pancreatic duct leak (n=7), prevention of post-ERCP pancreatitis (n=28), pseudocyst drainage (n=1), pancreas divisum (n=8), and pancreatic duct stricture without chronic pancreatitis (n=1). Standard Geenen® (Cook Endoscopy, Winston-Salem, NC, USA) pancreatic stents were placed in 53 patients (77.9%). The majority of stents (42, 61.8%) were completely occluded at the time of removal. Median time to stent occlusion was 35 days (95% CI: 30-40 days). Stent type, diameter, length, number of small side holes, and indication for placement were not predictive of subsequent stent occlusion. However, stents with at least 4 large, flange-associated side holes were 54% less likely to be clogged upon removal (HR=0.46, P=0.029). Conclusions Pancreatic stents are mostly occluded as early as 1 month after insertion. Larger side holes may prevent stents within the pancreas from becoming clogged.
Pancreatitis; Pancreatitis, Acute Necrotizing; Pancreatitis, Chronic; Stents
Over the last two decades, the placement of intraductal pancreatic endoprostheses during endoscopic retrograde cholangiopancreatography (ERCP) has become standard of care in select patients. Multiple studies have demonstrated the effectiveness of pancreatic stenting for the prevention of post-ERCP pancreatitis [1, 2] and the treatment of other conditions such as chronic pancreatitis [3, 4], pancreas divisum [5, 6], and pancreatic pseudocysts communicating with the main pancreatic duct [7, 8]. Stenting the pancreatic duct, however, is not without limitations or potential complications. These include the possibility of premature stent migration, ductal injury or perforation, and acute pancreatitis [9, 10, 11, 12]. Early rates of pancreatic stent occlusion have been documented in as soon as 9 weeks following insertion [13], and in nearly all patients with chronic pancreatitis after three months [14].
Despite the high prevalence of pancreatic stent occlusion, little is known about why it occurs, and less is known regarding the means of its prevention. Furthermore, the composition and design of pancreatic endoprostheses has remained relatively stagnant over the past 25 years. As such, the aims of our study were to estimate the frequency of pancreatic stent occlusion in a diverse group of patients at the time of removal, to study the accuracy of physicians’ prediction of occlusion during endoscopic assessment, and to determine the factors associated with stent occlusion.
Between August 2007 and July 2008, consecutive patients undergoing upper endoscopy for pancreatic stent removal as clinically indicated were prospectively enrolled at four separate tertiary referral centers. Permission to collect data was obtained from the institutional review board of each participating center.
Stent Removal and Testing
At the time of upper endoscopy, a standard sideviewing duodenoscope was used to inspect each stent prior to removal. Attending endoscopists were asked to predict whether or not the stent was occluded at the time of removal based on their visual inspection of the stent while still inside the pancreatic duct. A standard polypectomy snare was then used to grasp the stent at its most distal tip in effort to carefully withdraw it from the pancreatic duct without compromising stent integrity. All stents were removed through the working channel of the endoscope. Those with obvious damage, such as an excessively kinked tip or fracture, were excluded from further testing and withdrawn from the analysis.
Following removal, all stents were immediately pierced through the side of a water-filled Styrofoam® (Dow Chemical, Midland, MI, USA) cup with the duodenal flanges facing out in accordance with methods previously described by Ikenberry et al. [13] Care was taken to avoid impacting the proximal end of the stent tip with foam. Stents were observed for a total of two minutes during this “water-leak” test. Complete 100% occlusion was determined if there was no visible water flow from the duodenal end of the stent, the large flange-associated side holes, and the standard side holes along the length of the stent (Figure 1).
Figure 1. a. Example of an occluded pancreatic stent; the small drop of water at the base of the cup is due to leakage around the stent at the cup puncture site. b. Example of a patent pancreatic stent; notice water dripping from the larger, flange-associated side hole, as well as the smaller side hole in the middle of the stent.
After completion of the water-leak test, both patientrelated and stent-related factors were recorded. Patientrelated factors included the indication for stent placement and duration of therapy (days). Indication for stent placement was grouped in the following manner: 1) chronic pancreatitis; 2) prevention of post- ERCP pancreatitis; 3) pancreatic duct leak or stricture, pancreas divisum, and pancreatic pseudocyst. Stentrelated factors included stent type (standard pancreatic, single pigtail pancreatic, or standard biliary), stent length (3 cm, 4-5 cm, 7-8 cm, 9-15 cm), stent diameter (5Fr, 7Fr, 10Fr), the number of standard side holes (less than 3, 3-6, 7-10, more than 10) and large flangeassociated side holes (0-3, more than 3). Pancreatic stent occlusion was recorded (yes or no) by both the water-leak test and according to physician prediction at the time of endoscopic inspection.
Statistical analysis was performed using the SAS® system, version 9.1 (SAS Institute Inc., Cary, NC, USA). The median time to stent occlusion was estimated using the Kaplan-Meier method. A Cox regression was used to calculate hazard ratios (HRs) and their 95% confidence intervals (CIs). In a multivariate analysis, all variables were entered into the Cox regression model simultaneously and a stepwise selection was employed to find the best model fit. A two-tailed P value less than 0.05 was considered statistically significant.
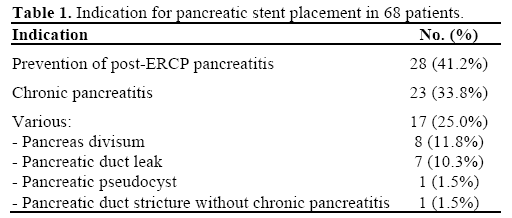
A total of 71 pancreatic stents were removed in 71 patients. Due to excessive damage upon removal, 3 stents were excluded from the study; thus allowing for a total of 68 stents to be analyzed. The indications for initial stent placement are listed in Table 1. The majority of patients had stents placed during ERCP for prevention of post-ERCP pancreatitis (28, 41.2%) and the treatment of chronic pancreatitis (23, 33.8%). Standard Geenen® (Cook Endoscopy, Winston-Salem, NC, USA) pancreatic stents were placed in 53/68 (77.9%) of patients. The remaining 15 (22.1%) stents were comprised of either Cotton-Leung® (Cook Endoscopy , Winston-Salem, NC, USA) biliary stents (n=6), or single pigtail Zimmon® (Cook Endoscopy, Winston-Salem, NC, USA) pancreatic stents (n=9) (Figure 2). The stent characteristics for all patients are shown in Table 2. The median time from stent insertion to stent removal was 27 d ays (range: 2-308 days, mean±SD: 35.1±40.7 days)

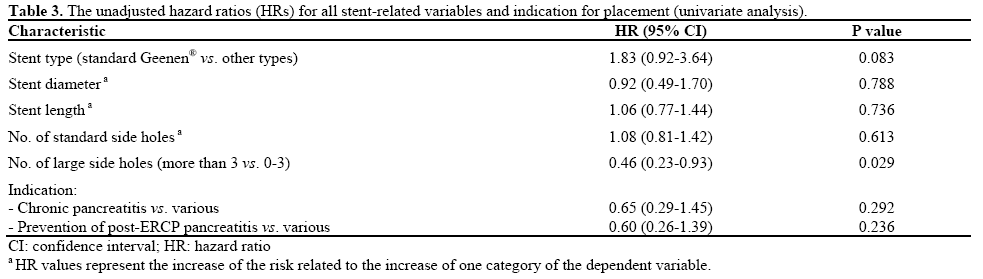
At the time of clinically indicated removal, 42/68 (61.8%) pancreatic stents were completely occluded according to the water-leak test. Figure 3 depicts the Kaplan-Meier survival analysis revealing a median time of 35 days to stent occlusion (95% CI: 30-40 days). Within the occluded group of pancreatic stents (n=42), physicians were correct in predicting complete stent obstruction at the time of endoscopic examination in 66.7% (28/42) of cases. In the group of nonoccluded pancreatic stents (n=26), physicians correctly predicted stent patency in 69.2% (18/26) of cases. The unadjusted HRs are reported in Table 3. Only the presence of more than 3 large flange-associated sided holes yielded a statistically significant impact on stent occlusion; that is, pancreatic stents with 4 or more large side holes were 54% less likely to be obstructed at the time of removal (HR=0.46, P=0.029; Table 3). There were no statistical associations between the remaining covariate factors and stent patency, including stent type, length, diameter, number of side holes and indication for placement. Similarly, the multivariate analysis did not yield significant independent associations between stent occlusion and the remaining covariates.
The practice of pancreatic stent placement during ERCP has become routine for certain conditions such as chronic pancreatitis, pancreas divisum, and the treatment of communicating pancreatic pseudocysts [3, 4, 5, 6, 7, 8]. Furthermore, pancreatic stents are now commonly placed in the main pancreatic duct during biliary ERCP as a means of preventing post-ERCP pancreatitis at times of difficult cannulation [15], or in certain high-risk populations such as patients with suspected sphincter of Oddi dysfunction [16]. Despite these practices, the reported rates of pancreatic stent occlusion are rather robust, and nearly 100% of stents are already clogged within 2-3 months of insertion [13, 14]. Identification of the factors associated with stent occlusion is clinically important because a clogged stent may be a poorly-functioning stent, and pancreatic stents themselves are not without potential complications including long term pancreatic ductal injury, stent migration, and acute pancreatitis [9, 10, 11, 12]. Nonetheless, there has been little change over the past 25 years in the overall design and composition of pancreatic endoprostheses.
Why do pancreatic stents clog? Farnbacher et al. examined the pancreatic stents of 49 patients with chronic pancreatitis following endoscopic removal [17]. Protein solubilization was achieved in effort to perform protein identification of the clogging material. More than one third of stents contained visible calcium carbonate calculi. Plant material, mucopolysaccharides, and crystals were present within the lumen of over 73% of stents. Dry weight of the clogging material correlated with stent diameter, but not with other stentrelated or patient-related criteria.
Farnbacher et al. used the results of this study in a similar group of patients with chronic pancreatitis [14]. A total of 47 patients (100 stents) had their pancreatic stents examined upon endoscopic removal. Major stent occlusion was defined as water flow reduction by 75% or more. In this homogeneous group of patients, four risk factors were associated with major stent occlusion: stent diameter greater than 8.5Fr, stent length greater than 8 cm, female gender, and exocrine insufficiency requiring the use of pancreatic enzyme supplementation.
In the current study, we aimed to identify the factors associated with complete pancreatic stent occlusion in a diverse group of patients. We examined the occlusion rates not only in patients with chronic pancreatitis (in which protein plugs and calcium calculi are expected to cause significant ductal obstruction) but also in those without chronic pancreatitis. We purposefully studied a heterogeneous population of patients as it more closely mimics what is encountered in the routine clinical practice of therapeutic endoscopy; that is, treating a variety of patients with different etiologies and spectra of diseases by means of pancreatic endotherapy. More than half (52.9%) the patients in our study underwent pancreatic stent placement for reasons other than the treatment of chronic pancreatitis. In a total of 68 patients, we found that 61.8% of pancreatic stents were completely occluded upon removal, and the median time to stent occlusion was 35 days after insertion. When the indication for stent placement was individually analyzed as a potential risk factor for stent occlusion, it was not found to be a significant determinant. Only the presence of more than 3 large, flange-associated side holes was associated with a reduced rate of stent occlusion. Lastly, we demonstrated that a physician’s ability to predict stent occlusion at the time of endoscopic removal is moderate at best; correctly predicting complete obstruction in only 66.7% of cases, and correctly predicting stent patency in 69.2% of cases.
Why would larger side holes be associated with lower rates of pancreatic stent occlusion? One explanation may be that stents with larger side holes allow for continued drainage of pancreatic secretions (despite being partially occluded) by means of capillary action created by the presence of larger outlet holes. In fact, stents without any small side holes, or lumen-less stents, have previously been shown to be associated with a larger surface area of flow, higher velocity of flow, and increased flow rates compared to conventional tubular stents [18]. The lumen-less “winged stent” (Viaduct® stent; GI Supply Inc., Camp Hill, PA, USA) was previously tested in a canine model to assess for short term pancreatic ductal injury [19]. The Viaduct® stent significantly ameliorated stent-induced changes to the main pancreatic duct, and was theorized to provide drainage of pancreatic secretions along the entire length of the stent. Imagine a pancreatic stent designed with numerous extra-large holes on all sides and along its entire length, thus appearing as the “skeleton” of a conventional tubular stent. With this in mind, it is plausible that this imaginary stent might also function as a lumen-less stent, or a “reverse” winged stent, with a unique design that provides increased flow rates with reduced rates of stent occlusion.
Of course, our study is not without limitations. Although the range of stent length was 3-15 cm in our analysis, only eight patients had pancreatic stents with a length greater than 8 cm. Furthermore, the overwhelming majority of patients had stent diameters of either 5Fr or 7Fr (66/68 patients). This may explain why our results did not corroborate those of Farnbacher et al. [14] with regards to higher rates of stent occlusion in larger, longer pancreatic stents. In addition, although our study contained a larger and more heterogeneous mix of patients, it lacks the overall size within each group of patients to directly compare the rates of stent occlusion in those with chronic pancreatitis versus all others.
Finally, three different types of pancreatic endoprostheses were used in the 68 patients enrolled in this study. Although all three types of stents were manufactured by the same company and made of similar composite material, this may have had an effect on our overall results. Individual differences in the long-term patency of each stent may not have been uncovered due to the relatively small number of patients in the study. Nonetheless, the overwhelming majority of stents used (78%) were of the same type (standard pancreatic stents; Geenen®; Cook Endoscopy, Winston-Salem, NC, USA).
Despite some of these limitations, this study demonstrates that pancreatic stents are associated with high rates of complete occlusion by the time they are endoscopically removed, even when placed for reasons other than chronic pancreatitis. Physicians are moderately proficient in predicting stent occlusion (or patency) at the time of removal. Large, flangeassociated side holes may be associated with reduced rates of stent clogging, and this concept warrants further investigation to assess for the possibility of improved rates of stent patency within the main pancreatic duct.
Financial disclosures None of the authors of this manuscript have any relevant financial disclosures or conflicts of interest to state