Review Article - (2023) Volume 9, Issue 2
Assessment of Pediatric Hidradenitis Suppurativa at a Regional Medical Center
Sarah N Rimmer1*,
John Miller1,
India Hill2,
Claudia Leonardi3 and
Deborah Hilton1
1Department of Dermatology, Louisiana State University School of Medicine, United States
2Department of Pediatrics, Children’s Hospital, United States
3Department of Public Health, Louisiana State University Health Sciences Center, United States
*Correspondence:
Sarah N Rimmer,
Department of Dermatology, Louisiana State University School of Medicine,
United States,
Email:
Received: 11-May-2023, Manuscript No. IPCPDR-23-16452;
Editor assigned: 15-May-2023, Pre QC No. IPCPDR-23-16452 (PQ);
Reviewed: 29-May-2023, QC No. IPCPDR-23-16452;
Revised: 05-Jun-2023, Manuscript No. IPCPDR-23-16452 (R);
Published:
12-Jun-2023, DOI: 10.36648/2472-0143.9.2.12
Abstract
Objective: Hidradenitis Suppurativa (HS) is a chronic, recurrent inflammatory disorder involving hair follicular occlusion, inflammation, and scarring. Few studies have been designed to characterize HS in pediatric patients. This retrospective study assessed the pediatric HS population at an academic medical center.
Methods: A retrospective chart review was performed of all encountersfor HS from April 2018 to July 2022 at Children’s Hospital in New Orleans, Louisiana. A total of 202 patients diagnosed with HS (ICD10=L73.2) were reviewed.
Results: Most patients identified as African American (72.4%). The mean age at HS onset was 12.1 years (SD: 1.9; range: 7-16); mean age of diagnosis was 13.0 years (SD: 1.9; range: 9-17). Family history of HS was not documented in 81.6% of pediatric patients. Pre-teen onset of disease (0-12 y) was recorded in 53.9% of patients. 50% of patients presented with Hurley stage I; 43.4%, stage II; and 6.6%, stage III. No association was found between Hurley stage at diagnosis and gender (p=0.610), race (p=0.603), or pre-teen onset (p=0.716). Eight patients were undergoing biologic therapy (infliximab, n=2; adalimumab, n=6), including 10.9% of females, 8.3% of males, 12.7% of African Americans, and 7.7% of Caucasians.
Conclusions: Family history is an important aspect of clinical history to ascertain in pediatric HS, as it is associated with early onset disease. Pediatric HS disproportionately affects non-Caucasian populations presenting with more severe disease. Cliniciansshould be aware of thistrend in caring for pediatric patients. 50% of patients on biologic therapy reported pre-teen onset of disease. In HS, early onset disease has been correlated with greater overall disease severity. Thus, more patients with early onset HS and likely moderate-to-severe disease will be initiated on biologic therapy in the future. It is crucial to detect pediatric HS in a timely manner, and counsel patients with pediatric HS on the possibility of biologic therapy.
Keywords
Hidradenitis suppurativa; Body mass index
Introduction
Hidradenitis Suppurativa (HS) is a chronic, recurrent inflammatory
disorder involving hair follicular occlusion, inflammation, and
scarring [1,2]. The disease is characterized clinically by episodes
of painful, inflamed, purulent nodules, abscesses, sinus tracts, and
scar formation at intertriginous areas [3,4]. HS is a chronic, debilitating disease that can have significant psychosocial impacts (i.e.
depression, anxiety) on the affected individual, as it is often recalcitrant to standard therapeutic options [4]. The average age of
onset is in the early 20’s while only 2%-3% of patients with HS report disease onset pre-pubertally (before 11 years old) [2,4,5]. HS
is more common in females than males by a ratio of 2:1 [6]. One
single-center study found that pre-pubescent onset of HS is more
likely to occur in males, while post-pubescent onset is more likely
in females [7]. Pediatric cases (<18 years old) of HS are rare and
can indicate endocrine axis anomaly, such as hormonal imbalance,
adrenal hyperplasia, and premature adrenarche [6,8-13]. Pediatric onset of disease is associated with a positive family history and
more widespread disease, and clinical disease manifestations are
also influenced by known risk factors such as smoking and Body
Mass Index (BMI) [2,14,15]. Case control studies indicate that pediatric patients with HS have higher rates of depression, anxiety,
acne vulgaris, obesity, metabolic syndrome, inflammatory bowel
disease, and Down syndrome [16-18].
Standard therapeutic options for the treatment of HS include topical
and systemic antibiotics, oral retinoids, hormonal therapy,
immunosuppressive drugs, biologic agents, and surgical excision
[4,19,20]. Early diagnosis and appropriate treatment of pediatric
HS is critical in minimizing the deleterious effects of the disease;
however, therapeutic management of HS in pediatric population
has been poorly studied. Mild cases are typically treated with topical antibiotics, while both topical and oral agents are used to treat
moderate disease. Modalities such as immunosuppressive agents,
biologic therapies, laser treatment, and surgery are reserved for
severe cases of HS [4,19,20]. Few studies, reviews, and practice
guidelines have been designed to characterize the demographics,
clinical characteristics, and therapeutic management of HS in pediatric patients. Given that research in pediatric HS is limited; this
retrospective study assessed the pediatric HS population at a large
regional academic medical center. The objective of this study is to
characterize the pediatric HS population and evaluate therapeutic
and surgical regimens currently in use.
Methods
A retrospective chart review was performed of all encounters
(i.e., emergency room visits, clinic visits with dermatologists and
non-dermatologists) for HS from April 2018 to July 2022 at Children’s Hospital in New Orleans, Louisiana (CHNOLA). A total of 202
patients diagnosed with HS (ICD10=L73.2) were reviewed. Patients
included in the analysis (n=77) were less than 18 years old at time
of HS diagnosis by a dermatologist or in 2 subsequent visits by a
non-dermatologist at CHNOLA. Diagnoses were confirmed if physical examination found typical lesions of HS at anatomic sites known
to commonly be affected by HS (e.g., axillae, buttocks, groin, and
inframammary region). Data extracted from the patients’ medical
chart included age, gender, race, payor status, age at diagnosis
of HS, age at onset of HS, disease severity (defined according to
Hurley stages; often documented; if not, based on examination
findings), anatomical distribution of disease at presentation, family history, and treatment regimen. Data was analyzed using the
SAS/STAT software, Version 9.4 of the SAS System for PC. Copyright
2014 SAS Institute Inc., Cary, NC. The association between Hurley
stage at diagnosis and gender, race, and pre-teen onset were assessed using either two-sided χ2 test or 2-sided Fisher exact test.
Significance was declared at P-value <0.05.
Results
Demographics
Patients’ characteristics are reported in Table 1. A total of 77 children with HS were included in study (64 female [84.2%], 12 male
[15.8%]). One patient was excluded because chart review revealed
inadequate documentation of medical history and clinical presentation. Most patients identified as African American (72.4%), and
the remaining patients identified as Caucasian (17.1%), other race
(6.6%), or declined to report their race (3.9%). Most pediatric patients with HS utilized Medicaid (84.2%), with the remaining payors being commercial insurance (10.5%), self-pay (2.6%), financial
assistance (1.3%), and Medicare (1.3%).
Table 1: Demographic features of pediatric patients with hidradenitis suppurativa (n=76)
| Item |
Mean (SD) |
| Age at onset, y |
12.1(1.9) |
| Age at diagnosis, y |
13.0(1.9) |
| Gender |
%(n) |
| Female |
84.2(64) |
| Male |
15.8(12) |
| Race |
0 |
| African American |
72.4(55) |
| Caucasian |
17.1(13) |
| Other |
6.6(5) |
| Patient Declined |
3.9(3) |
| Payor type |
0 |
| Medicaid |
84.2(64) |
| Commercial |
10.5(8) |
| Self-Pay |
2.6(2) |
| Medicare |
1.3(1) |
| Financial Assistance |
1.3(1) |
| Pre-teen (age 0 y-12 y) onset |
53.9(41) |
| Pre-teen onset in females (n=64) |
51.6(33) |
| Pre-teen onset in males (n=12) |
66.7(8) |
| Family history of HS |
0 |
| Yes |
17.1(13) |
| No |
1.3(1) |
| Not recorded |
81.6(62) |
| Hurley stage at diagnosis |
0 |
| I |
50.0(38) |
| II |
43.4(33) |
| III |
6.6(5) |
| SD=Standard Deviation. |
The mean age at HS onset was 12.1 years (Standard Deviation
[SD]:1.9; range: 7-16) and the mean age of diagnosis was 13.0
years (SD: 1.9; range: 9-17). Only 17.1% of patients (n=13) reported a family history of HS, 1.3% (n=1) of patients reported no family
history of HS, and family history was not documented in 81.6%
(n=62) of pediatric patients. Pre-teen onset of disease (0 y-12 y)
was recorded in 53.9% (n=41) of patients. Across gender, 51.6%
of females (n=33) and 66.7% of males (n=8) experienced preteen
onset of disease.
Disease Severity and Anatomic Distribution of
Sites
Overall, 50.0% of patients presented with Hurley stage I, followed
by 43.4% stage II (n=33), and 6.6% stage III (n=5). No significant association was found between Hurley stage at diagnosis and gender
(p=0.610), race (p=0.603), nor pre-teen onset (p=0.716) (Table 2).
Table 2: Univariable association between Hurley stage and patient characteristics (n=76).
| Item |
I(n=38) |
II(n=33) |
III(n=5) |
p-value |
| Gender |
-----------%(n)----------- |
0.61 |
| Female |
81.6(31) |
87.9(29) |
80.0(4) |
0 |
| Male |
18.4(7) |
12.1(4) |
20.0(1) |
0 |
| Race |
0 |
0.603 |
| African American |
68.4(26) |
75.8(25) |
80.0(4) |
0 |
| Caucasian |
21.1(8) |
15.2(5) |
0(0) |
0 |
| Other |
7.9(3) |
6.0(2) |
0(0) |
0 |
| Patient Declined |
2.6(1) |
3.0(1) |
20.0(1) |
0 |
| Pre-teen (age 0 yr-12 yr) onset |
0 |
0.716 |
| Yes |
42.1(16) |
48.5(16) |
60.0(3) |
0 |
| No |
57.9(22) |
51.5(17) |
40.0(2) |
0 |
The anatomic distribution of sites affected in pediatric patients
with HS is summarized in Table 3. Approximately one-third of patients were affected at two or more sites (38.2%, n=29). Of patients affected at >2 sites (38.2%), most were affected at 2 sites (58.6%).
Many patients, specifically 68.4% (n=52), presented with bilateral
involvement. The axilla was the most affected site (79.0%), followed
by the inguinal fold(s) (19.7%), inner thigh(s) (14.5%), and
mons pubis (13.2%).
Table 3: Anatomic Distribution of Sites in Pediatric Patients with Hidradenitis Suppurativa
| Item |
%(n) |
| Site location1 |
Total |
| Axilla |
79.0(60) |
| Inguinal fold (s) |
19.7(15) |
| Inner thigh (s) |
14.5(11) |
| Mons pubis |
13.2(10) |
| Chest |
9.2(7) |
| Labia |
7.9(6) |
| Buttocks |
5.3(4) |
| Inframammary fold (s) |
5.3(4) |
| Abdomen/panniculus |
4.0(3) |
| Intramammary fold (s) |
1.3(1) |
| Scalp |
1.3(1) |
| Breast |
1.3(1) |
| >2 Sites Involved |
38.2(29) |
| Bilateral involvement |
68.4(52) |
| 1Locations are not mutually exclusive |
Treatment Regimen
The topical, oral, and procedural treatment regimens utilized for
pediatric patients with hidradenitis suppurativa are summarized
in Figure 1 and Table 4. Many patients were treated with a single
oral antibiotic (67.1%), with doxycycline being the most frequently
utilized oral antibiotic (41.1%). Approximately 61.8% of patients
were treated with a topical antibiotic, with clindamycin being the
most frequently used (76.6%). A total of 59.2% of patients utilized
an antiseptic wash; chlorhexidine was the most common (73.3%).
Approximately 26.3% of patients utilized a regimen of combination oral antibiotics, of which clindamycin and rifampin was the
most common (65.0%). Patients also utilized other systemic medications (metformin, prednisone, sulfasalazine; 11.8%), biologic
therapy (10.5%), and hormonal treatment (spironolactone, oral
contraceptives; 9.2%). A total of 24 patients (31.7%) underwent a
procedure (intralesional corticosteroid, incision and drainage, and/
or excision) in the treatment of HS (Table 4). Of those, 25.0% were
treated with intralesional corticosteroids, 58.3% were treated with incision and drainage, and 37.5% were treated with excision.
Table 4: Topical, oral, and procedural treatment regimens for pediatric patients with hidradenitis supparativa (n=76)
| Item |
%(n) |
| Single oral antibiotic (n=51) |
0 |
| Doxycycline |
41.1(21) |
| Trimethoprim-Sulfamethoxazole |
21.6(11) |
| Clindamycin |
13.7(7) |
| Cephalexin |
5.9(3) |
| Minocycline |
5.9(3) |
| Amoxicillin |
3.9(2) |
| Rifampin |
3.9(2) |
| Dapsone |
2.0(1) |
| Erythromycin |
2.0(1) |
| Topical antibiotic (n=47) |
0 |
| Clindamycin |
76.6(36) |
| Mupirocin |
23.4(11) |
| Antiseptic wash (n=45) |
0 |
| Chlorhexidine |
73.3(33) |
| Benzoyl peroxide |
20.0(9) |
| Rubbing alcohol |
4.4(2) |
| Bleach baths |
2.2(1) |
| Combination Oral Antibiotics (n=20) |
0 |
| Clindamycin/Rifampin |
65.0(13) |
| Metronidazole/Moxifloxacin/Rifampin |
10.0(2) |
| Clindamycin/Doxycycline |
5.0(1) |
| Clindamycin/Trimethoprim-Sulfamethoxazole |
5.0(1) |
| Ampicillin-Sulbactam/Doxycycline |
5.0(1) |
| Other systemic medication (n=9) |
0 |
| Metformin |
81.8(9) |
| Prednisone |
9.1(1) |
| Sulfasalazine |
9.1(1) |
| Biologic Therapy (n=8) |
0 |
| Adalimumab |
80.0(8) |
| Infliximab |
20.0(2) |
| Hormonal Treatment (n=7) |
0 |
| Spironolactone |
71.4(5) |
| Oral contraceptive |
28.6(2) |
| Procedure (n=24) |
0 |
| Incision and drainage |
58.3(14) |
| Excision |
37.5(9) |
| Intralesional corticosteroids |
25.0(6) |
Biologic Therapy
Eight patients were undergoing biologic therapy (infliximab, n=2;
adalimumab, n=6), consisting of 10.9% of females (n=7), 8.3% of
males (n=1), 12.7% of African Americans (n=7), and 7.7% of Caucasians (n=1). These findings are summarized in Table 5. Only one
patient on biologic therapy reported a positive family history of
HS. Four patients on biologic therapy experienced pre-teen onset
of disease, and three were Hurley stage II at presentation, while
5 were Hurley stage III at presentation. Five patients on biologic
therapy (n=5) reported involved at >2 sites.
Table 5: Clinical characteristics of pediatric patients with hidradenitis supparativa utilizing biologic therapy (n=8)
| Gender |
n |
| Female |
7 |
| Males |
1 |
| Race |
0 |
| African American |
7 |
| Caucasian |
1 |
| Pre-teen Onset |
4 |
| Hurley Stage I |
0 |
| II |
3 |
| III |
5 |
| >2 involved sites |
5 |
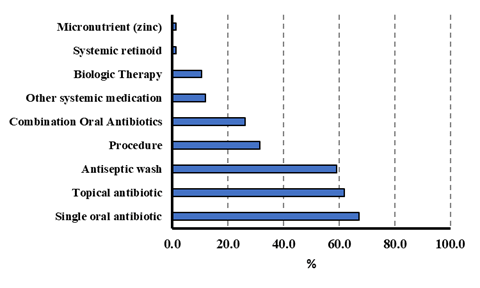
Figure 1: Treatment regimens for pediatric patients with hidradenitis supparativa (n=76)
Discussion
Demographics
In this study, pediatric HS was largely a diagnosis in young, African American women who utilize Medicaid, which is consistent
with prevalence estimates and other pediatric HS cohort studies
among children and adolescents [5,7,21,22]. Early onset HS has
been linked to female sex and this study showed a female preponderance for HS similar to gender ratios reported in the literature
(5:1 ratio vs 4:1, 4:1, 3:6:1, and 3:8:1) [5,21,23,24]. A small gap
existed between age at onset (12.1 y) and age at diagnosis (13.0
y). This diagnostic gap on the order of months is smaller than other pediatric cohort studies, except for Braunberger et al. who reported a diagnostic gap on the orders of months (11 years vs 11.9
years) as well [7,19,21]. In the general population, 35% of patients report a positive family history of HS [25]. Only 16.9% of patients
in our study reported a positive family history of HS; family history
was not reported in 81.6% of patients. This is an important aspect
of the clinical history to ascertain in pediatric HS, as family history
of HS is associated with early onset disease. Over half of patients
(53.9%) reported pre-teen onset of disease, consistent with other
pediatric cohort studies [19]. This underscores the need for various clinicians, i.e., primary care, emergency, endocrinology, dermatology, etc., to be aware of the prevalence and presentation of
HS in pediatric populations.
Disease Severity and Anatomic Distribution of
Sites
No univariable association was found between Hurley stage at
diagnosis and gender, race, and pre-teen onset. Despite these
findings, pediatric HS disproportionately affects non-Caucasian
populations presenting with more severe disease, and thus clinicians should be aware of this trend in caring for pediatric patients.
Larger studies are needed to further characterize pediatric HS and
its variable manifestations across gender and race.
Most patients presented with bilateral disease involvement
(68.4%), on par with similar pediatric cohort studies [21]. The axilla is typically the most involved site in pediatric HS across gender,
consistent with our study findings [19,21]. Continued awareness
of differences in HS presentation according to gender are crucial in
early diagnosis and minimizing long-term disease sequelae.
Treatment
Antibiotics, both topically and systemically, were used frequently
to treat pediatric patients with HS. While previously thought
that HS lesions are aseptic, recent research looking at HS lesions
challenges this notion [26-28]. A higher incidence of cutaneous
infection has been reported in children and adults with HS, and
Liy-Wong et al. suggests that traditional methods of skin swabbing
may be less useful in pediatric patients with HS [21,29]. Further
high-quality studies are needed to elucidate effective treatment
regimens and interventions for patients with pediatric HS. In our
study, most patients undergoing procedures for HS had an incision and drainage(s) performed. These procedures are typically
performed acutely in the setting of actively inflamed HS lesions.
Emergency and operating room visits are common throughout the
disease course of HS and can likely be mitigated in our pediatric
populations with earlier detection of disease, patient education
and appropriate treatment.
Biologic Therapy
While antiseptic washes and topical and oral antibiotics are the
mainstays of treatment, biologic therapies are being more frequently used in the treatment of moderate-to-severe pediatric
HS. Only adalimumab, a TNF-a inhibitor, has been approved by the
Food and Drug Administration (FDA) for the treatment of HS in children 12 years of age and older [30]. Additionally, studies in adults
with HS maintain highest treatment efficacy with TNF-a inhibitors
such as adalimumab and infliximab [31-33]. Of those patients in
our study being treated with biologic therapy, the majority were
African American females, consistent with the study population.
Family history of HS was nominal. Most patients were Hurley stage
III at time of presentation and had >2 involved sites. Interestingly, half of the patients on biologic therapy reported pre-teen onset of
disease [34]. In HS, early onset disease has been correlated with
greater overall disease severity [3,14,15]. It is thus probable that
more patients with early onset HS and thus likely moderate-to-severe
disease will be initiated on biologic therapy in the future. It
is crucial to detect pediatric HS in a timely manner, and counsel
patients with pediatric HS on the possibility of biologic therapy.
Limitations
Limitations of this work include its single-center, retrospective design.
Conclusion
The goal of this retrospective analysis was to characterize the
population of patients with pediatric-onset HS at a large academic
medical center. Characteristics of pediatric HS epidemiology, presentation, and treatment have traditionally been understudied.
Our results maintain that pediatric onset HS is a disease largely
affecting minority populations across race and gender. Early detection and effective treatment regimens are critical in mitigating
long-term sequelae of this debilitating disease, especially in pediatric populations. Varying manifestations of pediatric HS across
gender and race deserves further study, as well as the likelihood of
initiating biologic therapy in patients with pediatric onset disease.
Acknowledgement
None.
Conflict Of Interest
The author’s declared that they have no conflict of interest.
References
- Jemec GBE (2012) Hidradenitis suppurativa. N Engl J Med 66(2): 158-164.
[Crossref] [Google Scholar] [PubMed]
- Deckers IE, van der Zee HH, Prens EP (2014) Epidemiology of hidradenitis suppurativa: Prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep 3(1): 54-60.
[Crossref] [Google Scholar]
- Zouboulis CC, Benhadou F, Byrd AS, Chandran NS, Giamarellos-Bourboulis EJ, et al. (2020) What causes hidradenitis suppurativa? 15 years after. Exp Dermatol 29(12): 1154-1170.
[Crossref] [Google Scholar] [PubMed]
- Lam J, Krakowski AC, Friedlander SF (2007) Hidradenitis suppurativa (acne inversa): Management of a recalcitrant disease. Pediatr Dermatol 24(5): 465-473.
[Crossref] [Google Scholar] [PubMed]
- Garg A, Wertenteil S, Baltz R, Strunk A, Finelt N (2018) Prevalence estimates for hidradenitis suppurativa among children and adolescents in the United States: A gender and age-adjusted population analysis. J Invest Dermatol 138(10): 2152-2156.
[Crossref] [Google Scholar] [PubMed]
- Scheinfeld N (2015) Hidradenitis suppurativa in prepubescent and pubescent children. Clin Dermatol 33(3): 316-319.
[Crossref] [Google Scholar] [PubMed]
- Braunberger TL, Nicholson CL, Gold L, Nahhas AF, Jacobsen G, et al. (2018) Hidradenitis suppurativa in children: The henry ford experience. Pediatr Dermatol. 35(3):370-373.
[Crossref] [Google Scholar] [PubMed]
- Palmer RA, Keefe M (2001) Early-onset hidradenitis suppurativa. Clin Exp Dermatol 26(6): 501-503.
[Crossref] [Google Scholar] [PubMed]
- Prens LM, Rondags A, Volkering RJ, Janse IC, Politiek K, et al. (2019) The refined hurley classification: The inter-rater and intrarater reliability and face validity. Br J Dermatol 181(6): 1335-1337.
[Crossref] [Google Scholar] [PubMed]
- Prabhu G, Laddha P, Manglani M, Phiske M (2012) Hidradenitis suppurativa in a HIV-infected child. J Postgrad Med 58(3): 207-209.
[Crossref] [Google Scholar] [PubMed]
- Mengesha YM, Holcombe TC, Hansen RC (1999) Prepubertal hidradenitis suppurativa: Two case reports and review of the literature. Pediatr Dermatol 16(4): 292-296.
[Crossref] [Google Scholar] [PubMed]
- Feito-Rodriguez M, Sendagorta-Cudos E, Herranz-Pinto P, de Lucas-Laguna R (2009) Prepubertal hidradenitis suppurativa successfully treated with botulinum toxin A. Dermatol Surg Off Publ Am Soc Dermatol Surg Al 35(8): 1300-1302.
[Crossref] [Google Scholar] [PubMed]
- Lewis F, Messenger AG, Wales JK (1993) Hidradenitis suppurativa as a presenting feature of premature adrenarche. Br J Dermatol 129(4): 447-448.
[Crossref] [Google Scholar] [PubMed]
- Schrader AMR, Deckers IE, van der Zee HH, Boer J, Prens EP (2014) Hidradenitis suppurativa: A retrospective study of 846 dutch patients to identify factors associated with disease severity. J Am Acad Dermatol 71(3): 460-467.
[Crossref] [Google Scholar] [PubMed]
- Vaiopoulos AG, Nikolakis G, Zouboulis CC (2020) Hidradenitis suppurativa in paediatric patients: A retrospective monocentric study in Germany and review of the literature. J Eur Acad Dermatol Venereol 34(9): 2140-2146.
[Crossref] [Google Scholar] [PubMed]
- Tiri H, Jokelainen J, Timonen M, Tasanen K, Huilaja L (2018) Somatic and psychiatric comorbidities of hidradenitis suppurativa in children and adolescents. J Am Acad Dermatol 79(3): 514-519.
[Crossref] [Google Scholar] [PubMed]
- Wright S, Strunk A, Garg A (2020) New-onset depression among children, adolescents, and adults with hidradenitis suppurativa. J Am Acad Dermatol 83(5): 1360-1366.
[Crossref] [Google Scholar] [PubMed]
- Balgobind A, Finelt N, Strunk A, Garg A (2020) Association between obesity and hidradenitis suppurativa among children and adolescents: A population-based analysis in the United States. J Am Acad Dermatol 82(2): 502-504.
[Crossref] [Google Scholar] [PubMed]
- Seivright J, Collier E, Grogan T, Shih T, Hogeling M, et al. (2022) Pediatric hidradenitis suppurativa: Epidemiology, disease presentation, and treatments. J Dermatol Treat 33(4): 2391-2393.
[Crossref] [Google Scholar] [PubMed]
- Garcovich S, Fania L, Caposiena D (2022) Pediatric hidradenitis suppurativa: A cross-sectional study on clinical features and treatment approaches. J Cutan Med Surg 26(2): 127-134.
[Crossref] [Google Scholar] [PubMed]
- Liy-Wong C, Kim M, Kirkorian AY, Eichenfield LF, Diaz LZ, et al. (2021) Hidradenitis suppurativa in the pediatric population: An international, multicenter, retrospective, cross-sectional study of 481 pediatric patients. JAMA Dermatol 157(4): 385-391.
[Crossref] [Google Scholar] [PubMed]
- Riis PT, Saunte DM, Sigsgaard V, Villani AP, Guillem P, et al. (2020) Clinical characteristics of pediatric hidradenitis suppurativa: A cross-sectional multicenter study of 140 patients. Arch Dermatol Res 312(10): 715-724.
[Crossref] [Google Scholar] [PubMed]
- Molina-Leyva A, Cuenca-Barrales C (2019) Adolescent-onset hidradenitis suppurativa: Prevalence, risk factors and disease features. Dermatol Basel Switz 235(1): 45-50.
[Crossref] [Google Scholar] [PubMed]
- Dessinioti C, Tzanetakou V, Zisimou C, Kontochristopoulos G, Antoniou C (2018) A retrospective study of the characteristics of patients with early-onset compared to adult-onset hidradenitis suppurativa. Int J Dermatol 57(6): 687-691.
[Crossref] [Google Scholar] [PubMed]
- Ingram JR (2016) The Genetics of Hidradenitis Suppurativa. Dermatol Clin 34(1): 23-28.
[Crossref] [Google Scholar] [PubMed]
- Riverain-Gillet E, Guet-Revillet H, Jais JP, Ungeheuer MN, Duchatelet S, et al. (2020) The surface microbiome of clinically unaffected skinfolds in hidradenitis suppurativa: A cross-sectional culture-based and 16S rRNA gene amplicon sequencing study in 60 patients. J Invest Dermatol 140(9): 1847-1855.e6.
[Crossref] [Google Scholar] [PubMed]
- Naik HB, Jo JH, Paul M, Kong HH (2020) Skin microbiota perturbations are distinct and disease severity-dependent in hidradenitis suppurativa. J Invest Dermatol 140(4): 922-925.e3.
[Crossref] [Google Scholar] [PubMed]
- Langan EA, Recke A, Bokor-Billmann T, Billmann F, Kahle BK, et al. (2020) The role of the cutaneous microbiome in hidradenitis suppurativa-light at the end of the microbiological tunnel. Int J Mol Sci 21(4): 1205.
[Crossref] [Google Scholar] [PubMed]
- Lee HH, Patel KR, Singam V, Rastogi S, Silverberg JI (2020) Associations of cutaneous and extracutaneous infections with hidradenitis suppurativa in U.S. children and adults. Br J Dermatol 182(2): 327-334.
[Crossref] [Google Scholar] [PubMed]
- Gupta AK, Studholme C (2016) Adalimumab (humira) for the treatment of hidradenitis suppurativa. Skin Ther Lett 21(4): 1-4.
[Google Scholar] [PubMed]
- Lee RA, Eisen DB (2015) Treatment of hidradenitis suppurativa with biologic medications. J Am Acad Dermatol 73(5 Suppl 1): S82-S88.
[Crossref] [Google Scholar] [PubMed]
- Lim SYD, Oon HH (2019) Systematic review of immunomodulatory therapies for hidradenitis suppurativa. Biol Targets Ther 13: 53-78.
[Crossref] [Google Scholar] [PubMed]
- Wlodarek K, Ponikowska M, Matusiak L, Szepietowski JC (2019) Biologics for hidradenitis suppurativa: An update. Immunotherapy 11(1): 45-59.
[Crossref] [Google Scholar] [PubMed]
- Deckers IE, van der Zee HH, Boer J, Prens EP (2015) Correlation of early-onset hidradenitis suppurativa with stronger genetic susceptibility and more widespread involvement. J Am Acad Dermatol 72(3): 485-488.
[Crossref] [Google Scholar] [PubMed]
Citation: Rimmer SN, Miller J, Hill I, Leonardi C, Hilton D (2023) Assessment of Pediatric Hidradenitis Suppurativa at a Regional Medical Center. Clin Pediatr Dermatol. 9:12.
Copyright: © 2023 Rimmer SN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.