Keywords
Bacterial vaginosis; Nugent score; Clue cells; Amsel criteria; Whiff’s test; Northwest-Nigeria.
Introduction
The impact of many infectious diseases is complicated by pregnancy [1]. These infections are usually of considerable concern to clinicians because of the potential threats to the lives of the pregnant woman and that of her fetus [2]. Feto-maternal wellbeing in pregnancy and pregnancy outcome are certainly of great worry to the obstetrician and gynecologist. Bacterial vaginosis has the potential of causing late miscarriages, preterm births, preterm premature rupture of membranes, pelvic inflammatory disease, increased risk of human immunedeficiency virus infection, post-partum, post-abortal and postoperative endometritis. Detection, treatment and prevention of bacterial vaginosis may help improve perinatal and maternal morbidity and mortality. Bacteria vaginosis (BV) is the commonest cause of abnormal vaginal discharge in women of childbearing age and the most common infectious condition in women [3-5]. BV is particularly common in black women, especially in sub-Saharan Africa [6-9]. It is usually detected in 10-40% of women worldwide and is most prevalent among women who have multiple sexual partners; those of low income status and lower levels of education; among women who smoke cigarettes; in African-American women; and in women who do not use hormonal contraception [10]. Certain intra-vaginal practices have been linked with risk of acquiring Bacterial vaginosis. Intra-vaginal practices are common among sexually active women and have been described in several sub-Saharan African countries and parts of Asia and the United States of America [8]. These practices include vaginal wiping, vaginal washing (the most studied risk factor for BV) and inserting substances into the vagina. Vaginal washing may be by douching with a stream or jet of water or by using fingers to insert water, soap or variety of other substances into the vagina [8]. Women report using intra-vaginal practices for the purpose of genital hygiene, treatment of sexually transmitted diseases and to enhance sexual pleasure for male partners (dry sex). Although most women engage in these practices for perceived benefits, a number of studies have demonstrated potential harmful association with Human Immunodeficiency Virus 1 (HIV-1) infections, Sexually Transmitted Diseases (STDs), BV and other obstetric and gynecologic conditions such as Pelvic Inflammatory Disease (PIDs), decreased fertility and ectopic pregnancy [8]. Bacterial vaginosis has the potential of causing late miscarriages, preterm births, preterm premature rupture of membranes, pelvic inflammatory disease, increased risk of HIV infection, post-partum, post-abortal and post-operative endometritis [9]. Detection, treatment and prevention of bacterial vaginosis may help improve perinatal and maternal morbidity and mortality.
Materials and Method
The study was conducted between April and June 2008 at the Antenatal Clinic of Ahmadu Bello University Teaching Hospital, Shika, Zaria in Kaduna, one of the states in Northwest Nigeria. It was a cross sectional study combining the use of administered questionnaires with clinical examination and laboratory results. The interviewers completed the standardized baseline questionnaires containing information about patient demographics, reproductive profile, gynecologic history, previous adverse events during pregnancy and risk factors for BV. Laboratory analysis of samples taken from the study population was also carried out.
Kaduna State in Northwest Nigeria has a population of approximately 1.5 million (National Population Commission. Nigeria National Population Census 2005) and Zaria, one of the major cities in the State, has some prominent Federal institutions of higher learning and research. The Ahmadu Bello University Teaching Hospital (ABUTH) serves as a tertiary/ referral health facility for Zaria and its environs. Zaria occupies a portion of the high plains of Northern Nigeria, 652.6 m above sea level and some 950 km from the coast. It is located at 11°31 N, 7°42 E.
The Hausa-Fulani ethnic group constitutes more than 70% the population, and are mainly peasant farmers, predominantly of the Islamic faith. Other ethnic groups that live and work in Zaria include the Yorubas from the Southwest and the Igbos from the Southeast of the country. The study population were consenting pregnant women seeking antenatal care (first time attendees) at the ABUTH.
All consenting pregnant women encountered at the antenatal booking clinic of the health facility who met the inclusion criteria were recruited. The inclusion criteria were absence of systemic diseases such as diabetes mellitus, hypertension, renal disease; no prior history of placental abruption, uterine anomaly, incompetent cervix, twin pregnancy or prior antibiotic use in preceding two weeks before the study.
The antenatal booking clinic at the facility was held once a week with an average of forty women seen per week. The women were initially counselled to secure their consent for collection of various biological samples. All consecutive consenting pregnant women attending the antenatal clinic for the first time were recruited for the study until the desired sample size was attained.
Questionnaires were structured and pre-tested. Doctor and nurse interviewers specifically trained for the study screened each woman for study eligibility, explained the purpose and practice of the study and obtained informed consent. The same interviewers completed the standardized baseline questionnaires which contained information about patient demographics, reproductive profile, gynaecologic history, previous adverse effect in pregnancy and risk factors for BV.
The questionnaire was administered in a confidential location within the antenatal clinic by mostly female interviewers. Vaginal swabs for BV assessment were collected from clients using swab sticks which were immediately transported to the laboratory. At first, a clean, unlubricated speculum was introduced into the vagina and vaginal pH measured using pH strips. A sterile cotton swab was used to obtain material from the posterior vaginal fornix for vaginal smear. Wet smear of vaginal secretion from posterior fornix was diluted with normal saline, examined microscopically (x400) for identification of clue cells and bacteria morphocytes. These vaginal secretions (on wet smears) were also subjected to studies using Amsel and Nugent criteria. Each sample was also cultured for the offending organisms. The remainder of the prenatal care and BV screening was under the direction of the principal investigator.
The protocol and consent was approved by the ethical committee of ABUTH Zaria. Diagnoses were by Amsel criteria and Nugent score. By Amsel’s criteria, at least three of the four criteria should be present for diagnosis of Bacterial vaginosis to be confirmed. The criteria included (i) thin, white homogenous discharge, (ii) clue cells on microscopy, (iii) pH of vaginal fluid greater than 4.5, and (iv) release of fishy odor on adding alkali (10% potassium hydroxide) or positive Whiff’s test. The Nugent score estimated relative proportion of bacteria morphotypes to give a score between 0 and 10. Less than 4 was regarded as normal, 4-6 as intermediate, greater than 6 as indicative of the presence of Bacterial vaginosis. The Nugent score was the main diagnostic test.
| Variable |
Item |
Bacteria vaginosis |
χ2 (P-value) |
OR (95% CI) |
Total (%) |
| Positive |
Negative |
| Freq (%) |
Freq (%) |
| Age (years) |
20-24 |
8 (25.0) |
48 (25.5) |
0.04 (0.95) |
0.97 (0.41,2.31) |
57 (25.9) |
| 25-29 |
16 (50.0) |
58 (30.9) |
4.49 (0.03) |
2.24 (1.05, 4.79) |
74 (33.6) |
| 30-34 |
4 (12.5) |
26 (13.8) |
0.006 (0.94)* |
0.89 (0.29, 2.75) |
30 (13.6) |
| 35-39 |
4 (12.5) |
24 (12.8) |
0.06 (0.81)* |
0.98 (0.31, 3.03) |
27 (12.3) |
| ≥ 40 |
0 (0.0) |
32 (17.0) |
5.08 (0.02)* |
Undefined |
32 (14.6) |
| Educational status |
None |
1 (3.1) |
1 (0.5) |
0.18 (0.67)* |
6.03 (0.37, 98.98) |
2 (1.0) |
| Koranic |
10 (31.3) |
58 (30.9) |
0.002 (0.96) |
1.02 (0.45, 2.29) |
68 (30.9) |
| Primary |
9 (28.1) |
39 (20.7) |
0.87 (0.35) |
1.50 (0.64, 3.49) |
48 (21.8) |
| Secondary |
9 (28.1) |
63 (33.5) |
0.36 (0.55) |
0.78 (0.34, 1.78) |
72 (32.7) |
| Tertiary |
3 (9.4) |
27 (14.4) |
0.23 (0.63)* |
0.62 (0.18, 2.17) |
30 (13.6) |
| Occupational status |
Housewife |
12 (37.5) |
75 (39.9) |
0.07 (0.80) |
0.90 (0.42, 1.96) |
87 (39.5) |
| Business/trading |
7 (21.9) |
34 (18.1) |
0.26 (0.61) |
1.27 (0.51, 3.17) |
41 (18.6) |
| Civil servant |
1 (3.1) |
16 (8.5) |
0.49 (0.49)* |
0.35 (0.04, 2.71) |
17 (7.7) |
| Artisan |
7 (21.9) |
52 (27.7) |
0.47 (0.49) |
0.73 (0.30, 1.80) |
59 (26.8) |
| Student |
5 (15.6) |
11 (5.9) |
3.87 (0.04) |
2.30 (0.96, 9.24) |
16 (7.3) |
| Occupation of partner |
Civil servant |
12 (37.5) |
69 (36.7) |
0.01 (0.93) |
1.03 (0.48, 2.24) |
81 (36.8) |
| Farmer |
1 (3.1) |
12 (6.4) |
0.10 (0.75)* |
0.47 (0.06, 3.77) |
13 (5.9) |
| Business man |
3 (9.4) |
42 (22.3) |
2.08 (0.15)* |
0.36 (0.10, 1.24) |
45 (20.5) |
| Petty trader |
9 (28.1) |
14 (7.4) |
12.49 (0.0004) |
4.86 (1.89, 12.49) |
23 (10.4) |
| Artisan |
2 (6.3) |
25 (13.3) |
0.69 (0.41)* |
0.43 (0.10, 1.93) |
27 (12.3) |
| Parity |
0 |
3 (9.4) |
39 (20.7) |
1.61 (0.20)* |
0.40 (0.11, 1.37) |
42 (19.1) |
| 1 |
6 (18.8) |
30 (16.0) |
0.16 (0.69) |
1.22 (0.46, 3.21) |
36 (16.4) |
| 2-4 |
13 (40.6) |
71 (37.8) |
0.09 (0.76) |
1.13 (0.52, 2.42) |
84 (38.2) |
| 5 and more |
10 (31.3) |
48 (25.5) |
0.46 (0.50) |
1.33 (0.59, 3.00) |
58 (26.4) |
| Type of marriage |
Monogamous |
16 (50.0) |
125 (66.5) |
3.23 (0.07) |
0.5 (0.24, 1.07) |
141 (64.1) |
| Polygamous |
16 (50.0) |
63 (33.5) |
79 (35.9) |
| Total |
32 (14.5) |
188 (85.5) |
- |
- |
220 (100.0) |
Table 1 Socio-demographic characteristics of respondents,* Fisher’s exact test.
Reference group in each case referred to pregnant women who were confirmed to have Bacterial vaginosis. Data was analyzed using SPSS computer software version 15.0. Significance was considered at a p-value of <0.05. Data was presented in tables and figures.
Results
A total of 228 clients were initially recruited for the study but 220 (96.5%) of them completed it. Of these 220 clients, 32 (14.5%) were confirmed to have Bacterial vaginosis (BV) based on Nugent score (Table 1).
There was no significant statistical difference (t=-0.85, df=49.9, P-value=0.20) in the mean (± SD) age (in years) of clients with (25.5 ± 4.7) and without (26.3 ± 6.0) BV. The infection was more prevalent (16, 50.0%) among those aged 25-29 years who were 2.2 times more likely to present with BV than any other age group (χ²=4.49, P-value=0.03, OR=2.24, CI: 1.05, 4.79). Bacterial vaginosis was more prevalent among women who had only Koranic education (10, 31.3%), among occupational housewives (12, 37.5%) and among those with a parity of 2-4 (13, 40.6%).
| Variable |
Item |
Bacteria vaginalis |
Total (%) |
χ2 (P-value) |
OR (95% CI) |
| Positive |
Negative |
| Freq. (%) |
Freq. (%) |
| Gestational Age (weeks) |
1-13 |
1 (3.1) |
10 (5.3) |
11 (5.0) |
0.01 (0.93)* |
0.57 (0.07, 4.65) |
| 14-26 |
26 (81.3) |
146 (77.7) |
172 (78.2) |
0.21 (0.65) |
1.25 (0.48, 3.23) |
| 27-39 |
5 (15.6) |
32 (17.0) |
37 (16.8) |
0.04 (0.85) |
0.90 (0.32, 2.52) |
| 40 and over |
- |
- |
- |
- |
- |
| History of Vaginal Discharge |
Yes |
11 (34.4) |
74 (39.4) |
85 |
0.29 (0.59) |
0.81 (0.37, 1.77) |
| -Whitish discharge |
11 (100.0) |
45 (60.8) |
56 |
- |
- |
| -Thick |
6 (54.5) |
51 (68.9) |
57 |
- |
- |
| -Itching |
4 (36.4) |
21 (28.4) |
25 |
- |
- |
| -Rashes |
3 (27.3) |
17 (23.0) |
20 |
- |
- |
| Risk factors |
Douching |
-Remarkable Odor |
2 (18.2) |
10 (13.5) |
12 |
- |
- |
| Yes |
26 (81.3) |
162 (86.2) |
188 |
0.53 (0.47) |
0.70 (0.26, 1.85) |
| Hormonal contraceptive use |
No |
6 (18.8) |
26 (13.8) |
32 |
| Yes |
3 (9.4) |
35 (18.6) |
40 |
1.05 (0.31)* |
0.42 (0.13, 1.57) |
| HIV status |
No |
29 (90.6) |
153 (81.4) |
182 |
| Positive |
3 (9.4) |
7 (3.7) |
10 |
0.88 (0.35) |
2.63 (0.64, 10.76) |
| Negative |
29 (90.6) |
178 (94.7) |
207 |
| Total |
|
Unknown |
- |
3(1.6) |
3 |
- |
- |
| |
|
|
32 (14.5) |
188 (85.5) |
220 (100.0) |
- |
- |
Table 2 Prevalence of bacteria vaginosis by gestational age of index pregnancy, history of vaginal discharge and risk factors, *Fisher’s exact test.
Only 1 (3.1%) of the 32 BV positive clients presented for ANC in the first trimester (1-13 weeks) compared to 26 (81.3%) who presented in the second trimester (14-26 weeks) and 5 (15.6%) in the third trimester of pregnancy (Table 2). Though 11 (34.4%) of the BV positive and 74 (39.4%) of BV negative clients respectively complained of vaginal discharge, the overall prevalence of asymptomatic Bacterial vaginosis was 9.6% (21/220). Bacterial vaginosis was observed more among pregnant women who practiced douching (26, 81.3%) but not among those who used hormonal contraceptives (3, 9.4%) or those who were HIV positive (3, 9.4%). Of the 32 BV positive clients, 11 (34.4%) gave a history of vaginal discharge. All (11, 100.0%) had whitish discharge which was thick in 6 (54.5%) women. The discharge was associated with itching in 4 (36.4%) women, with rashes in 3 (27.3%) women and with remarkable odour only in 2 (18.2%) women (Figure 1).
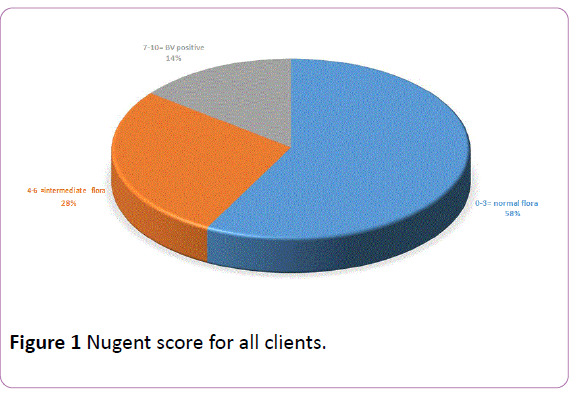
Figure 1: Nugent score for all clients.
Whiff’s test was positive for 14 (43.8%) BV positive and 99 (52.7%) BV negative clients with no significant statistical difference between these two groups (Table 3).
| Variable |
Item |
Bacteria vaginalis |
|
χ2 (P-value) |
OR (95% CI) |
| Positive |
Negative |
| Freq. (%) |
Freq. (%) |
| Characteristics |
pH ≤ 4.5 |
5 (15.6) |
10 (5.3) |
4.57 (0.03) |
3.30 (1.05, 10.38) |
| pH>4.5 |
27 (84.4) |
178 (94.7) |
| Clue cells |
Present |
17 (53.1) |
47 (25.0) |
10.49 (0.001) |
3.40 (1.58, 7.33) |
| Absent |
15 (46.9) |
141 (75.0) |
| Whiff test |
Positive |
14 (43.8) |
99 (52.7) |
0.87 (0.35) |
0.70 (0.32, 1.49) |
| Negative |
18 (56.3) |
89 (47.3) |
| Vaginal discharge on examination |
Yes |
18 (56.3) |
45 (23.9) |
0.78 (0.38) |
1.44 (0.64, 3.28) |
| Typical (thin, non-offensive, homogenous) |
8 (44.4) |
17 (37.8) |
1.63 (0.20) |
2.06 (0.68, 6.23) |
| |
Atypical |
8 (44.4) |
28 (62.2) |
| |
No |
14 (43.7) |
143 (76.1) |
- |
- |
Table 3 Prevalence of BV by components of the amsel criteria.
Pregnant women who were BV positive were about 1½ times more likely to present with thin, non-offensive homogenous discharge than those who were BV negative (χ2=0.78, Pvalue= 0.38, OR=1.44, 95% CI: 0.64, 3.28). In addition, BV positive clients were about twice more likely to present with typical thin, non-offensive homogenous vaginal discharge that BV negative clients (χ²=1.63, P-value=0.20, OR=2.06, 95% CI: 0.68, 6.23). Using Amsel’s clinical criteria, 25% had BV with a combination of i) positive Whiff’s test with 10% KOH, (ii) presence of clue cells on microscopy, (iii) vaginal pH>4.5 and (iv) thin, homogenous non-offensive discharge, thereby confirming the diagnosis of bacteria vaginosis The mean (± SD) pH of clients with bacterial vaginosis was 4.7 (0.5) while that of clients without BV was 4.8 (0.5) without any notable difference among the two. However, BV positive clients were 3.3 times more likely to have pH<4.5 than BV negative patients (χ²=4.57, P-value=0.03, OR=3.30, 95% CI: 1.05, 10.38) (Table 3). There was also a noteworthy variance in the proportion of BV positive clients among whom Clue cells were observed at microscopy (17, 53.1%) compared to those who were BV negative (47, 25%). Furthermore, patients who were BV positive were about 3½ times more likely to be Clue cell positive than those who were BV negative (χ²=10.49, Pvalue= 0.001, OR=3.40, 95% CI: 1.58, 7.33).
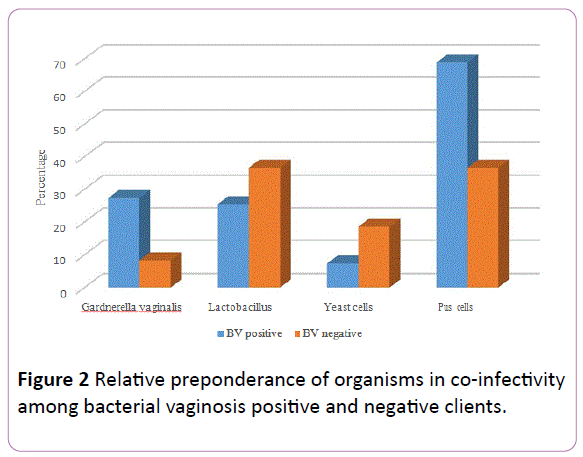
Figure 2: Relative preponderance of organisms in co-infectivity among bacterial vaginosis positive and negative clients.
Of the 32 clients who were diagnosed to be BV positive using Nugent score, only 12 (37.5%) were confirmed to be BV-positive while 20 (62.5%) were confirmed to be BV-negative using Amsel’s criteria. Using the Nugent score as the gold standard, Amsel criteria has a sensitivity of 37.5%, specificity of 70.7%, positive predictive value (PPV) of 17.9% and a negative predictive value (NPV) of 86.9% (Table 4).
Overall, 358 organisms were isolated from all the clients including 55 (15.4%) from the BV positive patients and 303 (84.6%) from BV negative patients (Table 5). Clients who were positive for BV were over four times more likely to have Gardnerella vaginalis co-infection (χ²=16.79, P-value=0.00004, OR=4.17, 95% CI: 2.03, 8.57) and were 1.15 times more likely to have Pus cells (χ²=0.23, P-value=0.63, OR=1.15, 95% CI: 0.64, 2.08) compared to those who were BV negative. Figure 2 shows the relative preponderance of these organisms.
| |
Nugent BV positive |
Nugent BV negative |
Sensitivity |
Specificity |
Positive Predictive Value |
Negative Predictive Value |
| Freq. (%) |
Freq. (%) |
| Amsel BV positive |
12 (37.5) |
55 (29.3) |
37.50% |
70.00% |
17.90% |
86.90% |
| Amsel BV negative |
20 (62.5) |
133 (70.7) |
Table 4 BV status by method of diagnosis.
Discussion
Bacteria Vaginosis (BV), the commonest lower female genital tract condition, causes a lot of problems in and outside of pregnancy [3-5]. In pregnancy, it causes late miscarriages, preterm labor, premature rupture of membranes, post-partum endometritis, low birth weight etc. [11-16]. All these impact on perinatal and maternal morbidity and mortality. BV is thus a significant infection in pregnancy.
| Organism |
BV positive (n=32) |
BV negative (n=188) |
Total |
χ2 (P-value) |
OR (95% CI) |
| Freq. (%) |
Freq. (%) |
| Gardnerella vaginalis |
15 (46.9) |
25 (13.3) |
40 (11.2) |
16.97 (0.00004) |
4.17 (2.03, 8.57) |
| Lactobacillus |
14 (43.8) |
111 (59.0) |
125 (34.9) |
2.56 (0.11) |
0.59 (0.31, 1.13) |
| Yeast cells |
4 (12.5) |
56 (29.8) |
60 (16.8) |
3.43 (0.06) |
0.35 (0.12, 1.00) |
| Pus cells |
22 (68.8) |
111 (59.0) |
133 (37.1) |
0.23 (0.63) |
1.15 (0.64, 2.08) |
| Total number of organisms |
55 |
303 |
358 |
- |
- |
Table 5: Organisms seen on microscopy according to presence of BV, NB: Some organisms were mixed on a single microscopy.
The prevalence of Bacterial vaginosis among this study population was 14.6% and this falls within 10-40% of the prevalence reported among women in the literature worldwide [11]. This is quite significant considering the complications of Bacterial vaginosis such as miscarriages, premature rupture of membranes and preterm birth, especially in an environment in sub-Saharan Africa, where facilities for neonatal resuscitation and intensive care are inadequate. Using polymerase chain reaction (PCR) method combined with Nugent score, Anukam et al. [4] also reported similar prevalence of BV (14.6%) among non-pregnant women in the southern part of Nigeria. This possibly indicates that pregnancy may not necessarily influence presence or absence of bacterial vaginosis. Certain key findings in this study need further discussion. The first is that Bacterial vaginosis was found to be most prevalent in certain sociodemographic spectrum.
It is interesting to note that this condition was not observed in pregnant women aged 40 years and above but was seen more in the age group of 25-29 years in consonance with what Turovskiy et al. [17] reported. Taking age group of 20-25 years into consideration, 75% of pregnant women consulting for ANC in this tertiary hospital would have been positive for Bacterial vaginosis. Early sexual debut and possible multiple sexual partners due to frequent divorce, are observed in Northern Nigeria and may be risk factors for BV as reported in some studies [18-21].
Bacteria vaginosis was not prevalent among clients with tertiary education but was more commonly found among those with little or no education. This finding agrees with what Harville et al. [11] reported that poor education and income are linked with higher risk of BV.
Bacteria vaginosis was rarer (9.4%) among women in their first pregnancy that among those who had had one child or more. In fact, the proportion of women with Bacterial vaginosis increased as parity increased till 4 after which the proportion declined. Also, more pregnant women in polygamous (16/79, 20.4%) than in monogamous (16/141, 11.3%) marriage presented with Bacterial vaginosis. One of the possible reasons for these situations is exposure to either multiple sexual partners and/or exposure to several heterosexual activities which are identified risk factor for BV [22].
Another key finding was that Bacterial vaginosis was more prevalent in the second and third trimester. The mean gestational age at which pregnant women in this study booked for ANC was 21 weeks. Trabet and Misra [23] reported increased likelihood of BV among women who have greater frequency of intercourse during the first trimester of pregnancy. On the other hand, after an 8 week follow-up from 14 weeks of gestation, Krauss-Silva et al. [24] observed a reversal to BV negative in about 40% of pregnant women who were initially positive. Presence or absence of BV at any stage of pregnancy seems to be a controversial issue. For example, a study reported that certain cytokines produced in mid-pregnancy were protective against BV, while others, such as IL6-174 G>C polymorphism increased the risk of developing BV [25]. The diagnosis of BV in the second trimester has been linked with high risk of preterm delivery and premature rupture of the membrane [26] though there may be other causes of preterm delivery and premature rupture of the membrane.
Incidentally, the proportion of pregnant women who practiced douching among the BV positive and BV negative clients was not significantly different, though among the 188 pregnant women who claimed they practiced douching, 26 (13.8%) were BV positive in contrast to the 6 (18.8%) of the 32 who claimed to not practice douching. Douching is a strong risk factor for BV, preterm birth, low-birth-weight infants, pelvic inflammatory disease, chlamydial infection, tubal pregnancy, higher rates of HIV transmission, and cervical cancer [27, 28].
One interesting finding was that BV positive patients were over 2½ times more likely to be HIV positive than BV negative patients. The prevalence of HIV among this cohort of pregnant women studied was 4.5% which agrees with the national HIV prevalence of 4.4% reported from the National Sentinel Survey [29]. This is in line with the documented suggestion that BV enhances heterosexual HIV transmission [30, 31].
The finding of typical vaginal discharge – thin, non-offensive and homogenous and non-offensive – in about 56% of the BV positive women correlates with the 58% reported by Srinivasan et al. [32]. The use of gram stained smear has been found to have higher sensitivity and specificity [33-35]. Using the Nugent score, only 14.6% had Bacterial vaginosis compared to 25% who had a combination of at least 3 of the 4 composite Amsel clinical criteria. Only 12 (37.5%) of the clients were BV positive by both Nugent score and Amsel clinical criteria [36]. Although Amsel criteria method is convenient and relatively inexpensive it is not always reliable. In this study, it had a low sensitivity compared to Nugent score. Kurki et al. [37] claimed that determination of vaginal pH is deficient in specificity since elevated vaginal pH may also depend on a variety of other conditions of the female lower genital tract.
Final major findings were the isolations of various organisms such as Gardnerella vaginalis more in BV positive (15/32, 46.9%) pregnant women similar to the 44.4% reported in The Gambia [6]. This was not surprising as this organism has been implicated as the causative agent of Bacterial vaginosis. For example, some authors [38, 39] proposed Gardnerella vaginalis biofilms as hazardous in BV pathogenesis and symptomatology. Lactobacillus spp., recorded less among BV positive pregnant women, was found to be one of most prevalent natural microbiota of the lower genital tract in women; others being L. crispatus, L. jensenii and L. iners [40-42], together creating a critical frontline guard against possible invading pathogens. Circulating hormones were thought to modulate the delicate symbiotic balance between Lactobacilli and the vaginal tract. Our finding of decreased concentration of Lactobacilli spp. was in accord with the explanation that BV, a poly microbial syndrome, resulted in milieu of decreased Lactobacilli spp. concentration and in increased pathogenic bacteria, including Gardnerella vaginalis [43, 44].
These findings have some clinical and practical implications. The high prevalence of BV among first time antenatal attendees (and by extension pregnant women in this study) reflects the potential negative effects BV can have on pregnancy and its outcome such as miscarriages, preterm births and increased risk of infection to the pregnant woman among other negative effects. The fact that most of the women in this study first presented for ANC relatively late (in their second trimester) when the damage may have been done is worrisome. Our study will therefore recommend early commencement of ANC and screening for BV at least in high risk women. Women in childbearing age should have access to health education on prevention of BV at school, or through radio and television. The clinical impact of the right measures taken as a result of the findings from this study will be a reduction of perinatal and maternal morbidity and mortality related to BV.
Conclusion
The overall prevalence of Bacterial vaginosis in pregnant women attending antenatal care for booking at ABUTH, Zaria is high and thus should be considered as an important condition in pregnant women by clinicians. The presence of clue cells on microscopy and isolation of Gardnerella vaginalis were significant indicators of presence of Bacterial vaginosis. There was no significant difference in mean pH of BV positive and BV negative patients and douching and other intra-vaginal practices were common practices among women in the study population. Screening for bacterial vaginosis preferably using the Nugent score, at least in high risk pregnant women may be worthwhile even if they are asymptomatic especially in the second trimester. A larger prospective study should be carried out to better demonstrate risk factors and possible adverse effects of Bacterial vaginosis.
Study Limitations
This study had some limitations that need explanation. Firstly, this was a facility-based study on a small non-representative sample of pregnant women. Therefore, extrapolating our findings to the general population may not be possible.
Secondly, first-time ANC clients typically presented during the later stages of pregnancy at ABUTH. Screening at earlier gestation would have been preferred. The study was unable to establish the temporality of BV infections, past pregnancy outcomes and risk factors for BV acquisition.
Thirdly, a prospective study to look at the effect of BV on pregnancy outcome was hampered by ethical issues and lack of consensus on whether to treat BV in pregnancy or not and the effect of either action. Equally of concern was the fact that follows up was difficult because patients/clients who enrolled at ABUTH ANC may not deliver in the hospital. Deliveries at home and elsewhere were still prevalent in Northern Nigeria when this study was carried out. These and other risk factors such as use of IUCD, previous STI, frequency of coitus and cigarette smoking could not be linked with presence of Bacterial vaginosis.
Lastly, the study was carried out in only one out of six geopolitical zones and several ecological locations such as the Atlantic Ocean coastline in the south and the mountainous regions on the eastern flank of the country. As such, conclusions based on findings may lack external validity.
References
- Gerberding JL (2004) Women and infectious diseases. Emerg Infect Dis: 1965-1967.
- Adachi K, Nielsen-Saines K, Klausner JD (2016) Chlamydia trachomatis infection in pregnancy: The global challenge of preventing adverse pregnancy and infant outcomes in Sub-Saharan Africa and Asia. Biomed Re Int: 9315757.
- Saunders S, Bocking A, Challis J, Reid G (2007) Effect of lactobacillus challenge on Gardnerella vaginalis biofilms. Colloids Surf B Biointerfaces 55: 138-42.
- Anukam KC, Osazuwa EO, Ahonkhai I, Reid G (2005) Association between absence of vaginal lactobacilli PCR products and nugent scores interpreted as bacteria vaginosis. Trop J Obstet Gynaecol 22: 103-107.
- Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, et al. (1994) Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. Br Med J 308: 295-298.
- Demba E, Morison L, van der Loeff MS, Awasana AA, Gooding E, et al. (2005) Bacterial vaginosis, vaginal flora patterns and vaginal hygiene practices in patients presenting with vaginal discharge syndrome in The Gambia, West Africa. BMC Infect Dis 5:12.
- Anukam KC, Osazuwa E, Osemene GI, Ehigiagbe F, Bruce AW, et al. (2006) Clinical study comparing probiotic lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacteria vaginosis. Microbes Infect 8: 2772-2776.
- Hassan WM, Lavreys L, Chohan V, Richardson BA, Mandaliya K, et al. (2006) Associations between intravaginal practices and vaginal vaginosis in Kenyan female sex workers without symptoms of vaginal infections. Sex Transm Dis.
- Yudin MH and Money DM (2008) Screening and management of bacterial vaginosis in pregnancy J Obstet Gynaecol Can 30:702-708
- Tann CJ, Mpairwe H, Morison L, Nassimu K, Hughes P, et al. (2006) Lack of effectiveness of syndromic management in targeting vaginal infections in pregnancy in Entebbe, Uganda. Sex Transm Infect 82: 285-289.
- Harville EW, Savitz DA, Dole N, Thorp JM Jr, Herring AH (2007) Psychological and biological markers of stress and bacteria vaginosis in pregnant women. BJOG 114: 216-23.
- Guaschino S, De Seta F, Piccoli M, Maso G, Alberico S (2006) Aetiology of preterm labour: Bacteria vaginosis. BJOG 113 :46-51.
- Hay PE (1998) Therapy of bacterial vaginosis. J Antimicrob Chemother 1998;41:6-9
- Centers for Disease Control and Prevention (1998) Guidelines for treatment of sexually transmitted diseases. MMWR 47:1-118.
- McGregor JA, French JI, Jones W (1994) Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase: Results of a controlled trial of topical clindamycin cream. Am J Obstet Gynecol 170: 1048-1059.
- Watson-Jones D, Weiss HA, Changalucha JM, Todd J, Gumodoka B, et al. (2007) Adverse birth outcomes in the United Republic of Tanzania- impact and prevention of maternal risk factors. Bull World Health Organ 85: 9-18.
- Turovskiy Y, Noll KS, Chikindas ML (2011) The etiology of bacterial vaginosis. J Appl Microbiol 110: 1105-1128.
- Fethers K, Twin J, Fairley CK, Fowkes FJI, Garland SM, et al. (2012) Bacterial vaginosis (BV) candidate bacteria: Associations with BV and behavioural practices in sexually-experienced and inexperienced women. PLoS ONE 7: e30633.
- Barbone F, Austin H, Louv WC (1990) A follow-up study of methods of contraception, sexual activity and rates of trichomoniasis, candidiasis and bacterial vaginosis. Am J Obstet Gynecol 163: 510-514.
- Moi H (1990) Prevalence of bacterial vaginosis and its association with genital infections, inflammation and contraceptive methods in women attending sexually transmitted disease and primary health clinics. Int J STD AIDS 1: 86-94.
- Paavonen J, Miettinen A, Stevens CE (1983) Mycoplasma hominis in non-specific vaginitis. Sex Transm Dis 45: 271-275.
- Fethers KA, Fairley CK, Hocking JS, Gurrin LC, Bradshaw CS (2008) Sexual risk factors and bacterial vaginosis: A systematic review and meta-analysis. Clin Infect Dis 47: 1426-1435.
- Trabert B, Misra DP (2007) Risk factors for bacterial vaginosis during pregnancy among African American women. Am J Obstet Gynecol 197:477e1-477e8.
- Krauss-Silva L, Almada-Horta A, Alves MB, Camacho KG, Moreira ME, et al. (2014) Basic vaginal pH, bacterial vaginosis and aerobic vaginitis: Prevalence in early pregnancy and risk of spontaneous preterm delivery, a prospective study in a low socioeconomic and multi-ethnic South American population. BMC Pregnancy Childbirth 14: 107.
- Goepfert AR, Varner M, Ward K, Macpherson C, Klebanoff M, et al. (2005) Differences in inflammatory cytokine and toll-like receptor genes and bacterial vaginosis in pregnancy. Am J Obstet Gynecol 193: 1478-1485.
- Purwar M, Ughade S, Bhagat B (2001) Bacterial vaginosis in early pregnancy and adverse pregnancy outcome. J Obstet Gynecol Res 27: 175-181.
- Cottrell BH (2003) Vaginal douching. J Obstet Gynecol Neonatal Nurs: 12-18.
- La Ruche G, Messou N, Ali-Napo L, Noba V, Faye-Ketté H, et al. (1999) Vaginal douching: Association with lower genital tract infections in African pregnant women. Sex Transm Dis 26: 191-196.
- Federal Ministry of Health (2008) Nigeria National HIV sero-prevalence sentinel survey (SPA Service Provision Assessment 2008).
- Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, et al. (1998) Bacterial vaginosis and disturbances of vaginal flora: Association with increased acquisition of HIV. Aids 12: 1699-1706.
- Cohen CR, Duerr A, Pruithithada N, Rugpao S, Hillier S, et al. (1995) AIDS. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand 9: 1093-1097.
- Srinivasan S, Morgan MT, Fiedler TL, Djukovic D, Hoffman NG, et al. (2015) Metabolic signatures of bacterial vaginosis. mBio 6: e00204-e00215.
- Nelson DB, Bellamy S, Nachamkin I, Ness RB, Macones GA, et al. (2007) First trimester bacterial vaginosis, individual microorganism levels and risk of second trimester pregnancy loss among urban women. Fertil Steril 88: 1396-1403. .
- Tolosa JE, Chaithongwongwatthana S, Daly S, Maw WW, Gaitan H, et al. (2006) The International Infections in Pregnancy (IIP) study: Variations in the prevalence of bacteria vaginosis and distribution of morphotypes in vaginal smears among pregnant women. Am J Obstet Gynecol 195: 1198-1204.
- Nugent RP, Krohn MA, Hillier SL (1991) Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29: 297-301.
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, et al. (1983) Non-specific vaginitis: Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74: 14-22.
- Kurki T, Sivonen A, Renkonen OV (1992) Bacterial vaginosis in early pregnancy and pregnancy outcome. Obstet Gynecol 80: 173-177.
- Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, et al. (2005) Adherent biofilms in bacterial vaginosis. Obstet Gynecol 106: 1013-1023.
- Josey WE and Schwebke JR (2008) The polymicrobial hypothesis of bacterial vaginosis causation: A reassessment. Int J STD AIDS 19: 152-154.
- Pavlova SI, Kilic AO, Kilic SS, So JS, Nader-Macias ME, et al. (2002) Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J Appl Microbiol 92: 451-459.
- Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, et al. (2004) Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology 150: 2565-573.
- Shi Y, Chen L, Tong J, Xu C (2009) Preliminary characterization of vaginal microbiota in healthy Chinese women using cultivation-independent methods. J Obstet Gynaecol Res 35: 525-532.
- Hill GB, Eschenbach DA, Holmes KK (1985) Bacteriology of the vagina. Scand J Urol Nephrol Suppl 86: 23-39.
- Hillier SL (1993) Diagnostic microbiology of bacterial vaginosis. Am J Obstet Gynecol 169: 455-459.