Research Article - (2022) Volume 23, Issue 6
Received: 12-May-2023, Manuscript No. IPP-23-15875; Editor assigned: 15-May-2023, Pre QC No. IPP-23-15875 (PQ); Reviewed: 29-May-2023, QC No. IPP-23-15875; Revised: 12-Jun-2023, Manuscript No. IPP-23-15875 (R); Published: 19-Jun-2023
Pancreatic cystic neoplasms account for approximately 10% of all pancreatic neoplasms and frequency of occurrence increases with age. Among cystic neoplasms, there are benign and malignant lesions. Malignant cysts are mainly diagnosed in the group of mucinous neoplastic cysts (containing mucinous fluid). The current guidelines for the management of asymptomatic pancreatic cystic lesions (consensus) is based mainly on the radiological features of PCL and Endoscopic Ultrasonography with Fine Needle Aspiration (EUS-FNA), which are controversial, mainly due to the limited sensitivity. The histopathological examination in each case concerned the entire lesion (pancreatic resections, local excisions) or a full walled sample (after drainage or palliative operations). However, as shown by the results in this study, the increased concentration of the CEA marker in the fluid does not correlate into an increased concentration in the blood. Although no differences were found in the mean blood concentration of the marker CEA and Ca 19-9 in the study groups, elevated values in the blood of these markers occurred in some cases of MCN, IPMN and non-neoplastic cysts. Tumor markers were determined by Electrohemiluminescence (ECL) using CEA specific biotinylated monoclonal antibodies and Ca 19-9 specific monoclonal antibodies. Mucinous neoplastic cysts, non-mucinous neoplastic cysts and non-neoplastic cysts do not differ in mean blood concentration of tumor markers Ca 19-9 and CEA. Since there is no single, widely used, non-invasive examination that differentiates mucinous neoplastic cysts with high sensitivity and specificity, researchers are moving towards the development of diagnostic systems combining multiple features, which significantly improved differentiation. According to experience of authors of this study, blood concentration of Ca 19-9 and CEA tumor markers should be considered as a rather supplement to the clinical data, imaging test, EUS-FNA and examinations of fluid from PCL.
Pancreatic cystic neoplasm, Endoscopic ultrasonography, Monoclonal antibodies, Blood concentration
Pancreatic cystic neoplasms account for approximately 10% of all pancreatic neoplasms and frequency of occurrence increases with age [1]. However, due to their cystic nature, pancreatic cystic neoplasms are also included to Pancreatic Cystic Lesions (PCL) and account for about 20% of PCL. The remaining 80% of PCL consists of non-neoplastic cysts, mainly represented by pseudocysts [2].
Among cystic neoplasms, there are benign and malignant lesions. Malignant cysts are mainly diagnosed in the group of mucinous neoplastic cysts (containing mucinous fluid). They are characterized by a high malignancy potential and are assumed to be precancerous lesions. Non-mucinous neoplastic cysts are mainly benign, and malignant tumors in this group are diagnosed very rarely. They are represented by SCN (Serous Cystic Neoplasms) and SPN (Solid Pseudopapillary Neoplasms) [3-5].
A significant clinical problem is the presence of pancreatic cancer precursors among cysts containing mucinous fluid and difficulties in diagnosis of these lesions [6]. The malignancy potential of mucinous neoplastic cysts: IPMN (Intraductal Papillary Mucinous Neoplasms) and MCN (Mucinous Cystic Neoplasms) cause the identification of patients with these tumors very important [7]. The current guidelines for the management of asymptomatic pancreatic cystic lesions (consensus) is based mainly on the radiological features of PCL and Endoscopic Ultrasonography with Fine Needle Aspiration (EUS-FNA), which are controversial, mainly due to the limited sensitivity [8-10]. For this reason, qualification for radical surgery is not easy (PCL malignancy risk assessment and correlation with perioperative risk) [11].
In diagnosis of pancreatic neoplasms (mainly pancreatic cancer) tumor markers play important role [12]. One of them is antigen Ca 19-9 tumor marker used in the diagnosis of Pancreatic Ductal Adenocarcinoma (PDAC) [13]. Its sensitivity in the diagnosis of PDAC is approx. 80%, specificity approx. 90% [14-16]. However, an increase in the blood concentration of this marker (>37 U/ml) is also observed in inflammatory diseases of the pancreas, in other neoplasms of the gastrointestinal tract (biliary tract, gall bladder, colon cancer), in mechanical jaundice and cholangitis, as well as in some patients with PCL [17,18]. Increased blood level of Ca 19-9 have been observed in some cases of malignant forms of IPMN and MCN [19,20].
In the differential diagnosis of PCL, it is much more useful to determine the CEA marker (carcinoembryonic antigen) in the fluid obtained from the cystic lesion. Increase in CEA concentration in fluid, with approx. 73% sensitivity, differentiates mucinous from non-mucinous neoplastic cysts, but does not differentiate between MCN and IPMN and between benign and malignant forms.
The aim of this study is to determine whether the serum concentration of Ca 19-9 and CEA tumor markers differentiates mucinous cystic neoplasms from other pancreatic cystic lesions.
This publication is a retrospective study. The material consisted of 202 patients with histopathologically confirmed pancreatic cysts (neoplastic cysts, non-neoplastic cysts) treated surgically in the years 2007-2021 at the general and transplant surgery department of the medical university of Lodz in Poland. The histopathological examination in each case concerned the entire lesion (pancreatic resections, local excisions) or a full-walled sample (after drainage or palliative operations). From this group, 134 patients were enrolled in the study according to the following criteria.
Inclusion criteria
•Patients with Ca 19-9 and CEA tumor markersmeasured in the blood up to 2 days before thesurgery.
•Patients who have no synchronous neoplasm inother organs.
Exclusion criteria
•Lack of examined concentrations of Ca 19-9 and/or CEA tumor markers in the blood up to 2 days before surgery.
•Patients with synchronous or other neoplasmof pancreas (e.g. PDAC, neuroendocrine tumor,secondary tumors) risk of false positive results.
According to these criteria, from the group of 202 patients, 68 patients were excluded from the study. In the excluded group 64 due to the lack of examination of Ca 19.9 and/or CEA tumor marker concentrations up to 2 days before surgery and 4 patients with other pancreatic neoplasms: 2 patients with IPMN with synchronous PDAC, 1 patient with malignant cystic neuroendocrine neoplasm (NET) and 1 patient with cystic metastasis of clear cell renal carcinoma. No patient had other active neoplastic disease at the time of treatment.
The patients were divided into 3 research groups in order to compare the differences between them in the concentration of the Ca 19-9 and CEA tumor marker in the blood.
The first group (n=31) consists of patients with Mucinous Neoplastic Cysts (MCN, IPMN), the second (n=24) patients with non-mucinous neoplastic cysts (SCN, SPN), while the third group (n=79) were patients with non-neoplastic cysts.
Tumor markers were determined by Electro-chemiluminescence (ECL) using CEA-specific biotinylated monoclonal antibodies and Ca 19-9 specific monoclonal antibodies.
Statistical tests were performed using Statistica® software. Standard statistical tools were used to perform descriptive statistics. Differences in the occurrence of particular types of pancreatic cystic lesions in correlation to gender was estimated using the Chi-square homogeneity test. Comparison of groups (mucinous neoplastic cysts, non-mucinous neoplastic cysts, non-neoplastic cysts) in terms of Ca 19-9 and CEA tumor markers concentrations was performed using the Kruskal-Wallis test. This test was used due to the unnatural distribution of the concentration of Ca 19-9 and CEA markers in the described groups and the size of the groups >2. The level of statistical significance for this test was p<0.05. The study of the distribution of the concentration of both markers in the described groups was performed with the Shapiro-Wilk test. In the case of this test, for a value of p<0.05, the hypothesis about the normality of distributions was rejected.
Mucinous neoplastic cysts of pancreas (MCN, IPMN, n=31) were both benign lesions adenomas (83.87%, n=26) as well as malignant lesions (16.13%, n=5).
Within MCN, malignant lesions (n=4) were Mucinous Cystadenocarcinomas MCN with invasive carcinoma, and within IPMN (n=1) Intraductal Pseudopapillary Mucinous adenocarcinoma IPMN with invasive carcinoma. MCN neoplasms were diagnosed more often in women (73.91% of women, 26.09% of men, p<0.001). These data are summarized.
All non-mucinous neoplastic cysts (SCN and SPN, n=24) were benign. SCN tumors were the most common non-mucinous pancreatic neoplasms. They accounted for 83.33% of non-mucinous neoplastic cysts and 14.93% of all PCL. SCN tumors occurred more often in women (85% of women, 15% of men, p<0.001), and the mean age was 63.15 years. The mean age for the remaining, much rarer non-mucinous neoplastic cysts of the pancreas SPN, represented by Solid Pseudopapillary Tumors (SPT), was 37 years.
Of the non-neoplastic cysts, pseudocysts where the most common. They accounted for 89.87% of non-neoplastic cysts and 52.99% of all PCL included in the study. Non-neoplastic cysts occurred more frequently in men (men 63.29%, women 36.81%, p=0.001). Number of patients, mean age, malignancy and the frequency of occurrence of all PCL are presented.
The mean concentration of the Ca 19-9 marker for mucinous neoplastic cysts was 13.86 U/ml (11.42 U/ml for IPMN, 14.7 U/ml for MCN). It did not differ significantly (p=0.24) compared to non-mucinous neoplastic cysts 10.94 U/ml and non-neoplastic cysts 10.25 U/ml (Figure 1).
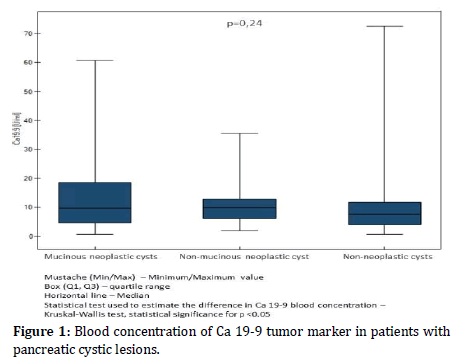
Figure 1: Blood concentration of Ca 19-9 tumor marker in patients with pancreatic cystic lesions.
Similarly, in the described groups, the difference was not observed in the concentration of the CEA marker (p=0.11). In the case of mucinous neoplastic cysts of the pancreas, the mean CEA marker concentration was 2.62 ng/ml, in patients with non-mucinous neoplastic cysts 1.77 ng/ml and in patients with a non-neoplastic cyst 2.45 ng/ml (Figure 2). Mean concentrations of markers, minimum and maximum values and standard deviations are summarized.
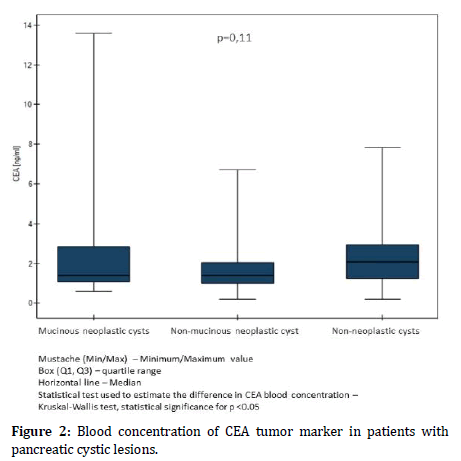
Figure 2: Blood concentration of CEA tumor marker in patients with pancreatic cystic lesions.
So far, the role of testing the concentration of the CEA marker in the fluid obtained from the cyst in the differentiation of mucinous and non-mucinous cysts has been proven (sensitivity approx. 75%, specificity approx. 85%) 21,24. However, as shown by the results in this study, the increased concentration of the CEA marker in the fluid does not correlate into an increased concentration in the blood. Although no differences were found in the mean blood concentration of the marker CEA and Ca 19-9 in the study groups, elevated values in the blood of these markers occurred in some cases of MCN, IPMN and non-neoplastic cysts. Elevation of these markers in the blood was not observed in any of the non-mucinous neoplasms (SCN and SPN).
Among all PCL included in the study, an increase in the concentration of CEA in the blood (>5 ng/ml) occurred in 12.5% of MCN, 13% of IPMN and 10% of non-neoplastic cysts.
A similar percentage of increase in Ca 19-9 blood concentration was observed in 8.7% of MCN, 13% of IPMN and 2.5% of non-neoplastic cysts. For this reason, the increase in the blood concentration of the CEA or Ca 19-9 marker should be considered as a risk factor for the occurrence of a mucinous neoplastic cyst.
It is stated that increase in the blood concentration of Ca 19-9 or CEA in IPMN and MCN tumors is a risk factor for their malignancy. The mean concentration of the Ca 19-9 marker in the blood for malignant mucinous cysts was 29.7 U/ml and was significantly higher (p=0.02) than in the case of benign mucinous cysts 10.8 U/ml, but due to the small amount of malignant cystic neoplasms in this study (n=5), the results are not reliable.
Since there is no single, widely used, non-invasive examination that differentiates mucinous neoplastic cysts with high sensitivity and specificity, researchers are moving towards the development of diagnostic systems combining multiple features, which significantly improved differentiation. One of them is a CompCyst ®25. Data obtained in CompCyst system include: Clinical symptoms, computed tomography features and molecular markers in fluid (mutations and aberrations in genes: BRAF, CDKN2A, CTNNB1, GNAS, KRAS, NRAS, PIK3CA, RNF43, SMAD4, TP53, and VHL). The authors emphasize that due to the high sensitivity in diagnosis of mucinous cyst (92% to 96%) and specificity (94%-100%) this approach reduces the risk of missing a pancreatic cyst requiring surgical treatment and reduces the number of unnecessary operations. At the moment, such a diagnostic system is not widely used due to the limitations such as the cost and invasiveness of EUS-FNA25.
According to experience of authors of this study, blood concentration of Ca 19-9 and CEA tumor markers should be considered as a rather supplement to the clinical data, imaging test, EUS-FNA and examinations of fluid from PCL.
Mucinous neoplastic cysts, non-mucinous neoplastic cysts and non-neoplastic cysts do not differ in mean blood concentration of tumor markers Ca 19-9 and CEA.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Wlazlak M, Durczynski A, Hogendorf P, Grzasiak O, Strzelczyk J. Ca 19-9 and Cea Tumor Markers in Blood and Pancreatic Cyst. An Analysis of 134 Patients with Histopathologically Verified Pancreatic Cystic Neoplasms and Non-Neoplastic Cysts. JOP. J Pancreas. (2023) 24:808.
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.