Research Article - (2023) Volume 9, Issue 2
Cathelicidin Protects the Brain from Mitochondrial DNA Damage in Health, but not Following Septic Shock
Denise Frediani Barbeiro1,
Suely Kubo Ariga1,
Hermes Vieira Barbeiro1,
Nadja C. Souza-Pinto2 and
Fabiano Pinheiro da Silva1*
1Department of Clinical Emergencies, University of Sao Paulo, Brazil
2Department of Biochemistry, University of Sao Paulo, Brazil
*Correspondence:
Fabiano Pinheiro da Silva,
Department of Clinical Emergencies, University of Sao Paulo,
Brazil,
Email:
Received: 31-Jan-2023, Manuscript No. IPJICC-23-15590;
Editor assigned: 02-Feb-2023, Pre QC No. IPJICC-23-15590 (PQ);
Reviewed: 16-Feb-2023, QC No. IPJICC-23-15590;
Revised: 21-Feb-2023, Manuscript No. IPJICC-23-15590 (R);
Published:
28-Feb-2023, DOI: 10.35248/2471-8505.23.9.011
Abstract
Recent discoveries have demonstrated that mitochondria play a critical role in innate immune signaling. By the other hand, immune responses may lead to mitochondrial deregulation. Cathelicidins play a critical role in innate immunity, promoting poorly understood cellular responses that may enhance or inhibit several signaling pathways, depending on the health conditions and subjacent microenvironment. Here, we investigated the role of CRAMP, the murine cathelicidin, in healthy mice and following experimental sepsis. We found that sepsis induces significant mitochondrial DNA damage in the prefrontal cortex and that cathelicidin protects the brain from this kind of damage in healthy animals, but not following septic shock.
Keywords
Cathelicidins; Innate immunity; Brain; DNA damage; Sepsis; Inflammation
Introduction
On one hand, we know that DNA is highly susceptible to
chemical damage. The DNA replication and repair machinery,
moreover, make mistakes. On the other hand, cells possess a
sophisticated DNA repair system [1]. Mitochondria are particularly
susceptible to DNA damage, since they act as the cellular
powerhouses and have to deal with a permanent production
of Reactive Oxygen Species (ROS). It is true that an imbalance
between ROS generation and cellular system’s ability for clearance,
promotes damage to lipids, proteins and nucleic acids
throughout the cell [2]. Mitochondrial DNA, however, besides
its close contact with the respiratory chain, is not protected by
histones or a nuclear envelope, becoming an easy target to oxidative
lesions. Finally, it is important to cite that besides small,
the mitochondrial genome encodes 13 proteins that take part
in the oxidative phosphorylation complex and mutations in
such genes can also serve to increase ROS cellular levels [3].
Such factors contribute to a high mutagenesis rate [3]. ROS
accumulation can lead to DNA base modifications, deletions,
strand breaks and crosslinks. Oxidative stress and DNA damage
have been linked with multiple chronic conditions, such as cancer,
neurodegenerative processes, diabetes, cardiovascular diseases,
chronic inflammatory diseases and aging [4-10]. Here,
we hypothesize that DNA damage may also be an important
phenomenon to the pathophysiology of sepsis, an acute condition
characterized by deregulation of the immune response
and intense systemic inflammation. Since the brain is particularly
susceptible in sepsis and mitochondrial and immune functions
are tightly linked [11,12], we decided to investigate DNA
damage in the prefrontal cortex of wild-type and CRAMP-deficient
mice, submitted or not to experimental sepsis. Cathelin-derived antimicrobial peptide (CRAMP) is an antimicrobial
peptide that modulates several aspects of the immune response
[13]. It is the only cathelicidin in rodents and its counterpart
in humans is named LL-37. Cathelicidins are a family of
antimicrobial peptides able to directly kill a range of pathogens,
including bacteria, protozoa and virus. Despite that, cathelicidins
also play a dual role in the immune-inflammatory response
through intriguing and poorly understood mechanisms. Indeed,
depending on the disease and cellular context, cathelicidins can
stimulate or inhibit the immune-inflammatory system [14].
Materials and Methods
Cecal Ligation and Puncture
Young (8 weeks old) and aged (18 months old) male CRAMP−/−
mice on a C57 BL/6 genetic background and their matched WT
controls were purchased from The Jackson Laboratory (ME,
USA). We induced peritonitis using the model of cecal ligation
and puncture (CLP), as previously described [15]. Briefly, animals
were anesthetized and the cecum ligated and punctured
twice with a 21 G needle, allowing fecal material to be released
into the peritoneal cavity. Animals were sacrificed 24 hours after
the surgery and plasma and tissue samples of the brain (prefrontal
cortex) were collected for further analyses.
DNA Extraction
The samples were prepared according to the instructions in the
Qiagen DNeasy Blood and Tissue kit (#69506 Qiagen). DNA was
eluted in 100μ l of elution buffer. The concentration of genomic
DNA was determinutesed using Nanoview (GE). Samples were
diluted in elution buffer for the PCR assays (6 ng/μL).
PCR Reaction
Amplification of a 16540 bp segment of mitochondrial DNA was
performed using Accu Prime Taq DNA Polymerase High Fidelity
(#12346-086 Invitrogen) with forward and reverse primers (10
μM); Total DNA (30 ng); Buffer II 10X (5 μL); Taq DNA Polymerase
(0,2μL) and H2O to complete 50μL. Primers sequences were:
Forward, 5‘-TGAGGCCAAATATCATTCTGAGGGGC-3’ and reverse,
5‘-TTTCATCATGCGGGAGATGTTGGATGG-3’. PCR conditions were
(1) 94°C for 30 seconds; (2) 60°C for 30 seconds, and (3) 68°C for
18 minutes (26 cycles). Amplification of a 140 bp segment of
mitochondrial DNA was performed using Taq DNA Polymerase
(#10342-053 Invitrogen) with dNTP (10 mM); MgCl2 (50 mM);
Forward and reverse primers (10μM); Total DNA (3 ng); Buffer
10X (5μL); Taq DNA Polymerase (0,2μL) and H2O to complete
50μL. Primers sequences were: Forward, 5‘-ACTTACGCAAAGGCCCCAACG-
3’ and reverse, 5‘-GAGCTAAGGTCGGGGCGGTG-3’.
PCR conditions were 94°C for 3 minutes; (1) 94°C for 45 seconds;
(2) 56°C for 30 seconds and (3) 72°C for 1 minute (22 cycles).
Statistical Analysis
Results were analyzed using Kruskal-Wallis test, followed by
Mann-Whitney U test with Bonferroni adjustment. Results are
shown in boxplots. All analyses were performed using R statistical
software (www.r-project.org). A p-value <0.05 was considered
significant.
Results
CRAMP protects the brain from mtDNA damage under normal
conditions, but not following experimental sepsis. Both young
wild-type mice and young CRAMP-deficient submitted to experimental
sepsis showed significant mtDNA damage in the brain,
when compared to the control groups (p<0.001 and 0.003, respectively).
Secondly, the presence of CRAMP protected the
brain of wild-type mice from further mtDNA damage under normal
conditions, but not following sepsis (p=0.011). Aged mice
were used as positive controls. As expected, aged mice exhibited
more DNA damage in the brain than young mice.
Discussion
It is widely accepted that oxidative stress has a crucial role in sepsis evolution [16,17]. Mitochondrial dysfunction and several ultrastructural changes have been reported in many organs during sepsis [18-22]. It has even been postulated that mitochondrial dysfunction plays a central role in the pathogenesis of the Multiple Organ Dysfunction Syndrome (MODS) that frequently follows the course of septic shock and many other inflammatory catastrophes [23,24]. The topic, however, remains controversial. Some authors argue that the studies are still very heterogeneous and inconsistent [25]. DNA damage, for example, as far as we know, had never been investigated in sepsis. Here, we show that mitochondrial DNA damage is aggravated in the brain of wild-type and CRAMP-deficient mice, 24 hours after the induction of experimental sepsis, when compared to the control groups, putting in evidence that sepsis induces significant mtDNA damage [26]. Mitochondrial DNA damage, moreover, is more severe in the brain of healthy CRAMP KO mice, when compared to healthy wild-type mice, showing that CRAMP protects from mitochondrial DNA damage under normal conditions (Figure 1).
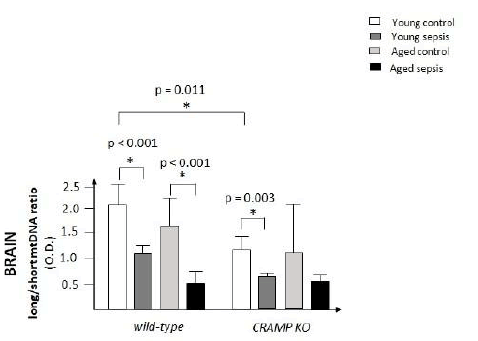
Figure 1: Ratio of mtDNA damage in the brain (prefrontal cortex) of wild-type and CRAMP-deficient mice under normal conditions and following experimental sepsis (n=5-9 animals per group).
Conclusion
We believe that the increase in mtDNA damage in CRAMP-deficient
mice, detected only under normal conditions, but not
following sepsis, occurred because sepsis induces such a robust inflammatory response that the protective effects of CRAMP
became subtle in this situation. Septic encephalopathy patients,
thus, may benefit from a targeted therapy directed to restore
mitochondrial integrity.
Acknowledgement
None.
Conflict of Interest
The authors have no relevant financial or non-financial interests
to disclose.
Funding
FPS is supported by FAPESP, the Sao Paulo Research Foundation
(grant #2020/03905-2020/03908), and by CNPq, the National
Council for Scientific and Technological Development (grant
#303924/2018-303924/2017).
Avaialability of Data and Material
The detailed results of our experiments are available upon request.
Author's Contributions
SKA performed the in vivo experiments. DFB and HVB performed
the in vitro experiments. FPS and NCSP conceived the
project. FPS analyzed the data and wrote the first draft. FPS and
NCSP wrote the final manuscript.
Ethics Approval
Protocols were in accordance with the University of Sao Paulo
Faculty of Medicine Ethical Committee (project number
953/2017).
References
- Chatterjee N, Walker GC (2017) Mechanisms of DNA damage, repair and mutagenesis. Environ Mol Mutagen 58(5): 235-263.
[Crossref] [Google Scholar] [Pubmed]
- Valera AM, Canto C (2018) Mitochondrial stress management: A dynamic journey. Cell Stress 2(10): 253-274.
[Crossref] [Google Scholar] [Pubmed]
- Sharma P, Sampath H (2019) Mitochondrial DNA integrity: Role in health and disease. Cells 8(2): 100.
[Crossref] [Google Scholar] [Pubmed]
- Sosa V, Teresa M, Rosa S, Rosanna P, Hiroshi K, et al. (2013) Oxidative stress and cancer: An overview. Ageing Res Rev 12(1): 376-90.
[Crossref] [Google Scholar] [Pubmed]
- Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360(1): 201-205.
[Crossref] [Google Scholar] [Pubmed]
- Burgos ME, Zaida AJ, Aranzazu MM, Francesca I, Irene E, et al. (2019) Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J Clin Med 8(9): 1385.
[Crossref] [Google Scholar] [Pubmed]
- Peng W, Guoding C, Yiping X, Jinna C, Peng W, et al. (2019) Mitochondrial dysfunction in atherosclerosis. DNA Cell Biol 38(7): 597-606.
[Crossref] [Google Scholar] [Pubmed]
- Potz BA, Sellke FW, Abid MR (2016) Endothelial ROS and impaired myocardial oxygen consumption in sepsis-induced cardiac dysfunction. J Intensive Crit Care 2(1): 20.
[Crossref] [Google Scholar] [Pubmed]
- Kirkham PA, Barnes PJ (2013) Oxidative stress in COPD. Chest 144(1): 266-273.
[Crossref] [Google Scholar] [Pubmed]
- Belenguer VA, Francisco JTS, Juan AAZ, Marta MR (2019) Oxidative stress and exceptional human longevity: Systematic review. Free Radic Biol Med 149: 51-63.
[Crossref] [Google Scholar] [Pubmed]
- Chen Y, Zhou Z, Min W (2018) Mitochondria, oxidative stress and innate immunity. Front Physiol 9: 1487.
[Crossref] [Google Scholar] [Pubmed]
- Park DW, Zmijewski JW (2017) Mitochondrial dysfunction and immune cell metabolism in sepsis. Infect Chemother 49(1): 10-21.
[Crossref] [Google Scholar] [Pubmed]
- Fabiano PS, Marcel CCM (2012) Antimicrobial peptides: Clinical relevance and therapeutic implications. Peptides 36(2): 308-314.
[Crossref] [Google Scholar] [Pubmed]
- Pinheiro da Silva F, Machado MC (2017) The dual role of cathelicidins in systemic inflammation. Immunol Lett. 182: 57-60.
[Crossref] [Google Scholar] [Pubmed]
- Wichterman KA, Baue AE, Chaudry IH (1980) Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res 29(2): 189-201.
[Crossref] [Google Scholar] [Pubmed]
- Mantzarlis K, Tsolaki V, Zakynthinos E (2017) Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid Med Cell Longev 2017: 5985209.
[Crossref] [Google Scholar] [Pubmed]
- Nagar H, Piao S, Kim CS (2018) Role of mitochondrial oxidative stress in sepsis. Acute Crit Care 33(2): 65-72.
[Crossref] [Google Scholar] [Pubmed]
- Aki T, Unuma K, Uemura K (2017) Emerging roles of mitochondria and autophagy in liver injury during sepsis. Cell Stress 1(2): 79-89.
[Crossref] [Google Scholar] [Pubmed]
- Hu Q, Jianan R, Huajian R, Jie W, Xiuwen W, et al. (2018) Urinary mitochondrial DNA identifies renal dysfunction and mitochondrial damage in sepsis-induced acute kidney injury. Oxid Med Cell Longev 2018: 8074936.
[Crossref] [Google Scholar] [Pubmed]
- Pan P, Wang X, Liu D (2018) The potential mechanism of mitochondrial dysfunction in septic cardiomyopathy. J Int Med Res 46(6): 2157-2169.
[Crossref] [Google Scholar] [Pubmed]
- Gonzalez AS, Elguero ME, Finocchietto P, Holod S, Romorini L, et al. (2014) Abnormal mitochondrial fusion-fission balance contributes to the progression of experimental sepsis. Free Radic Res 48(7): 769-783.
[Crossref] [Google Scholar] [Pubmed]
- Parikh SM, Yuan Y, Liyu H, Chengyuan T, Ming Z, et al. (2015) Mitochondrial function and disturbances in the septic kidney. Semin Nephrol 35(1): 108-119.
[Crossref] [Google Scholar] [Pubmed]
- Pool R, Gomez H, Kellum JA (2018) Mechanisms of organ dysfunction in sepsis. Crit Care Clin 34(1): 63-80.
[Crossref] [Google Scholar] [Pubmed]
- Wu Y, Yao YM, Lu ZQ (2019) Mitochondrial quality control mechanisms as potential therapeutic targets in sepsis-induced multiple organ failure. J Mol Med (Berl) 97(4): 451-462.
[Crossref] [Google Scholar] [Pubmed]
- Jeger V, Siamak D, Stephan MJ, Jukka T (2013) Mitochondrial function in sepsis. Eur J Clin Invest 43(5): 532-542.
[Crossref] [Google Scholar] [Pubmed]
- Fabiano PS, Marcel CCM (2015) Personalized Medicine for Sepsis. Am J Med Sci 350(5): 409-413.
[Crossref] [Google Scholar] [Pubmed]
Citation: Barbeiro DF, Ariga SK, Barbeiro HV, Pinto NCS, Pinheiro da Silva F (2023) Cathelicidin Protects the Brain from
Mitochondrial DNA Damage in Health, but not Following Septic Shock. J Intensive Crit Care. 9:011.
Copyright: & copy; 2023 Barbeiro DF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution
License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and
source are credited.


