- (2006) Volume 7, Issue 3
Ulf Petersson, Anders Borgstrom
Department of Surgery, Malmo University Hospital, Lund University. Malmo, Sweden
Received:13 February 2006 Accepted: 28 February 2006
Context All work on human trypsinogen activation peptide (TAP) in acute pancreatitis has been carried out with the same assay. Despite the extensive use of this original TAP assay, there is no characterization of the TAPlike immunoreactivity measured. Objective The aims of this study were to develop an additional TAP assay and to attempt to characterize the TAP-like immunoreactivity found in the urine of patients with acute pancreatitis. Methods :Antibodies against the human TAP were prepared using the whole octapeptide APFD4K, conjugated at its N-terminal end. Characterization of the immunoreactivity measured with these assays was performed using gel filtration of human pancreatic juice before and after activation of trypsinogen with enterokinase. Results After activation of the pancreatic juice, there was a large initial increase in immunoreactive TAP and a decrease 6-24 hours later. Using our antiserum, we found low levels of immunoreactive TAP in urine from patients with acute pancreatitis, although many of these samples contained high levels of immunoreactive TAP when tested with the commercially available TAP kit® (Biotrin). The pentapeptide D4K, used as a standard in the Biotrin kit, showed much lower immunoreactivity than the synthetic octapeptide APFD4K in our assay. The octapeptide, however, reacted similarly to D4K in the Biotrin kit assay. Conclusion Our antibody prepared against the synthetic octapeptide APFD4K is directed against the N-terminal part of the octapeptide and does not recognize the pentapeptide D4K. Immunoreactive TAP in urine in acute pancreatitis is mainly composed of the Cterminal pentapeptide, D4K.
Biological Markers; Pancreatitis, Acute Necrotizing; Trypsinogen; trypsinogen activation peptide
TAP: trypsinogen activation peptide
Trypsinogen is one of the most predominant zymogens in pancreatic juice. Activation of trypsinogen by enterokinase in the duodenum [1] is the initial step in the normal activation of pancreatic digestive enzymes. Premature activation of the proenzymes within the pancreas was suggested as the cause of acute pancreatitis as early as 1896 [2]. Today, the pathophysiological importance of trypsinogen activation and trypsin activity in acute pancreatitis [3, 4, 5, 6, 7, 8, 9, 10] is generally accepted, even if the initiation and timing of this activation, as well as its relationship to other inflammatory reactions, is still under debate. One explanation for this is that trypsin activity has been difficult to measure in biological fluids, as trypsin activated in the pancreatic tissue or in the circulation is momentarily bound, and thereby inactivated, by protease inhibitors. When trypsinogen is activated, an N-terminal 8-amino acid long peptide is split off [11]. An assay for analysis of this trypsinogen activation peptide (TAP) was first described in 1988 [12], and is one way of monitoring trypsinogen activation. Since then, a limited number of papers on human acute pancreatitis have been published [8, 9, 13, 14, 15, 16, 17]. Measurement of TAP levels in urine has been suggested as a possible way of predicting the severity of an attack of acute pancreatitis early in the course of the disease [8, 9, 14, 16].
The five C-terminal amino acids of TAP are highly conserved phylogenetically. An assay with antibodies directed at this part of TAP can therefore be used in many different species of experimental animals. A large number of papers on trypsinogen activation in different models of experimental acute pancreatitis have been published using this method [5, 18, 19].
All the work on TAP in acute pancreatitis has been carried out with the same assay. No alternative assay has been available for confirmation of the results. Despite the extensive use of the original TAP assay, no characterization of the TAP-like immunoreactivity measured with this method has been presented. The aims of this study were to develop an additional TAP assay and to attempt to characterize the TAP-like immunoreactivity found in the urine of patients with acute pancreatitis.
Peptides
Synthetic human TAP (trypsinogen activation peptide) APFDDDDK (APFD4K) was obtained from Euro-Diagnostica (Malmö, Sweden). This peptide was used as a standard in our assay. Two other peptides YAPFD4K (Tyr-TAP) and CAPFD4K (Cys-TAP) were also purchased from the same source. The Cys-TAP peptide was used for immunization. The Tyr-TAP peptide was labelled with I125 using the chloramine-T method. The monoiodinated peptide was purified by HPLC using a C18 column, and used as a tracer in the assay. Porcine enterokinase was obtained from the Sigma Chemical Company (St. Louis. MI, USA).
Immunization Procedure
The Cys-TAP peptide was conjugated at its N-terminus to bovine serum albumin using maleimidobenzoyl-N-hydroxysuccimide and rabbits were immunized by multiple subcutaneous injections of this conjugate in Freund’s incomplete adjuvant. We obtained three different polyclonal antisera which reacted with I125Tyr-TAP when diluted more than 1/1,000. In the following study, only one of these antibodies was used (designated as 9801).
Activation of Pancreatic Juice
Human pancreatic juice devoid of trypsin activity was obtained by drainage of the main pancreatic duct after pancreatic head resections in 3 patients (2 men and 1 female aged 45, 67 and 68 years) The juice was kept at -20°C for up to 12 months before use. For use, 1.8 mL of pancreatic juice was treated with 200 μL of enterokinase (1 mg/mL in 0.05 mol/L Tris-HCl buffer, pH 7.4, containing 0.05 mol/L CaCl2) and incubated at room temperature. One hundred μL was withdrawn at time zero and after 1 h, 2 h, 4 h, 6 h, 10 h, 15 h, 24 h and 48 h. These 100 μL samples were boiled for 15 min and diluted to 1 mL in the buffer described above. The samples were then stored at 4°C for up to 2 days before analysis.
Gel Chromatography
Gel filtration was performed on a Sephadex G-50 column, 0.9x60 cm, in the buffer described above. The column was eluted at 3 mL/h and the eluate was collected in 0.6 mL fractions.
Radioimmunoassays
The assay buffer used was 0.05 mol/L Tris- HCl, pH 7.4 containing 0.15 mol/L NaCl, 0.005 mol/L EDTA and 2 g/L bovine serum albumin. Samples (100 μL) diluted in assay buffer were incubated for 16 h at 4°C with 200 μL of I125Tyr-TAP (about 20,000 cpm) in assay buffer and 200 μL rabbit anti octapeptide APFD4K serum (9801) diluted 1/1,500 in assay buffer. Parallel incubation of synthetic TAP diluted in assay buffer (0.1-10 nmol/L) served as standards. Free and bound radioactivities were then differentiated by means of a second antibody precipitation step using a decanting suspension (with sheep anti-rabbit IgG antibody) (Pharmacia, Uppsala, Sweden). After four hours of incubation, the sample was centrifuged at 2,500 g for 15 min, the supernatant was decanted and the radioactivity of the precipitate was measured in a gammacounter. The sensitivity of the assay was estimated to be 0.1 nmol/L.
Biotrin TAP Assay
This assay was purchased from Biotrin International (Dublin, Ireland). It was run according to the manufacturer’s instructions. The standard used in this assay is the pentapeptide D4 K. The production and specificity of the polyclonal antiserum has been described [12].
Stability of the Octapeptide APFD4K (TAP) in Serum, Plasma and Urine
Synthetic TAP (APFD4K) was mixed with either fresh serum, fresh serum boiled for 15 min, fresh EDTA plasma, or fresh urine (all obtained from one healthy subject), or with Tris-HCl buffer, pH 7.4, containing 0.05 mol/L CaCl2 (see above). One sample was withdrawn immediately from each mixture and boiled. The mixtures were then incubated at room temperature and further samples were withdrawn at different time intervals for up to 48 h and boiled. TAP-like immunoreactivity was then measured using our assay.
Urine Samples
Urine samples were obtained from 14 patients with acute pancreatitis. (8 males, 6 females; mean±SD age: 62±11 years. The samples were stored at 4°C for up to 18 hours after sampling and then frozen at -20°C for up to 6 months before analysis. The diagnosis was based on acute abdominal pain of less than 72 hours duration, with at least a threefold elevation of the serum amylase level.
ETHICS
The local animal welfare committee (m318- 95) accepted the immunization procedure. The procedure for drainage of the main pancreatic duct after pancreatic head resections was approved by the local ethics committee (LU Dnr 616/2004); written consent was obtained from all patients. Finally, the local ethical committee (LU-00- 47) approved the urine samples obtained from patients with acute pancreatitis. The study protocol conforms to the ethical guidelines of the "World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects" adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, as revised in Tokyo 2004.
Data are reported as mean±SD. The Wilcoxon matched-pairs test was applied. A two-tailed P value less than 0.05 was considered statistically significant. The SPSS for Windows (Version 13.0) statistical package was used to perform the statistical analysis.
Radioimmunoassay for Immunoreactive Trypsinogen Activation Peptide (irTAP)
Figure 1 shows the pattern of immunoreactivity for the synthetic octapeptide APFD4K and the Biotrin pentapeptide D4K using the tracer I125Tyr-TAP and the antiserum produced in this study. Our antibody is very sensitive to the octapeptide and was found to recognize this intact TAP at concentrations as low as 0.1-0.2 nmol/L while reactivity to the pentapeptide was found to be much lower, the sensitivity for this peptide being only around 5-10 nmol/L. Figure 2 shows the corresponding ELISA results for the Biotrin kit antibody using the kit. This assay is about twice as sensitive to the pentapeptide than to the octapeptide. The sensitivity for the octapeptide in this assay is in the range 0.5-1.0 nmol/L. Boiling of the octapeptide did not affect the immunoreactivity in either of the assays (data not shown).
Ir-TAP in Human Pancreatic Juice
The concentrations of ir-TAP in pancreatic juice before and after activation of trypsinogen with enterokinase, measured with both our assay and the Biotrin assay, are shown in Figure 3. All samples were boiled before analysis. There are clear differences in the time curves obtained with the two assays. Before activation, the Biotrin assay could not detect any immunoreactivity while our assay found approximately 0.02 μmol/L of ir-TAP. Using our assay, maximal immunoreactivity was seen one hour after activation (about 11 μmol/L) while maximal immunoreactivity with the Biotrin assay was found after 4 hours (about 14 μmol/L). After the initial activation, the immunoreactivity decreased rapidly in our assay while that measured by the Biotrin assay was more stable. A reasonable interpretation of the data would be that the immunoreactive material measured with our assay is more labile than that measured using the Biotrin assay.
Characterization of ir-TAP in Pancreatic Juice Using Gel Chromatography
Figure 4 shows a characterization of immunoreactive TAP in pancreatic juice using gel chromatography and our radioimmunoassay. Samples were boiled before gel filtration to avoid autoactivation during chromatography. No immunoreactivity was noted before activation with enterokinase. After activation, a peak of ir- TAP was found in the vicinity of fractions 56 and 57. Gel chromatography of the synthetic octapeptide APFD4K showed that this peptide is eluted in exactly the same volume (results not shown). This experiment was not performed using the Biotrin assay or the D4K standard.
Figure 4. Elution profiles after gel filtration of immunoreactive TAP in pancreatic juice, before and after activation by enterokinase. Gel filtration was performed on a Sephadex G-50 column (0.9x60 cm). Immunoreactive TAP was measured in each fraction using radioimmunoassay based on the 9801 antibody.
Ir-TAP in Urine Obtained from Patients with Acute Pancreatitis
Urine samples obtained from patients with acute pancreatitis were found to contain no or only small amounts of TAP-like immunoreactivity when measured with our radioimmunoassay while significantly larger amounts of immunoreactivity were recorded using the ELISA assay from Biotrin (Table 1). Only one sample (patient 4) contained clearly elevated levels of irTAP as measured with our assay (antiserum 9801).
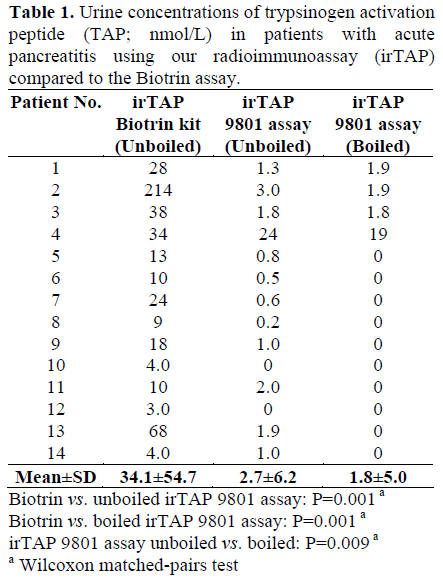
Characterization of ir-TAP in Urine from Patients with Acute Pancreatitis Using Gel Chromatography
Radioimmunoassay
The elution profile after gel chromatography of the TAP-like immunoreactivity in urine from patient 4, as measured by our assay, is shown in Figure 5 (unboiled sample). Most of the immunoreactivity was recovered in one peak (peaking in fraction 58) which was eluted in a volume corresponding to the elution profile of the activation peptide found in activated pancreatic juice (Figure 4). A smaller peak was also detected, with the elution volume corresponding to the size of trypsinogen (with peak in fraction 30). This peak was completely abolished when the urine sample was boiled before gel filtration while the peak corresponding to the activation peptide was unchanged (Figure 5, boiled).
Figure 5. Elution profile (after gel filtration) of immunoreactive TAP from the urine of a patient with acute pancreatitis, as measured by radioimmunoassay with 9801 antibodies before and after boiling. Gel filtration was performed on a Sephadex G-50 column (0.9x60 cm). Arrows indicate the elution volume of trypsinogen (Tg) and the elution volume of the octapeptide APFD4K (TAP)
Biotrin Assay
The elution profile after gel filtration of the TAP-like immunoreactivity in urine from patient 2, using the assay from Biotrin, is shown in Figure 6. All immunoreactivity was eluted in a similar or perhaps slightly larger volume (with peak in fraction 60) than the activation peptide seen in Figure 5, indicating that this immunoreactivity corresponded to the same size of molecule. Testing these fractions with our assay did not allow detection of immunoreactivity in any fraction.
Stability of Synthetic APFD4K in Serum, Plasma and Urine
The synthetic octapeptide loses its immunoreactivity in serum within hours, as shown in Figure 7. However, the immunoreactivity appears to be more stable in urine. The loss of immunoreactivity in serum can be prevented by boiling the sample or by adding EDTA, which chelates divalent cations. This experiment was not performed with the Biotrin assay.
Figure 7. Stability of TAP in serum plasma and urine. Synthetic APFD4K was incubated in fresh serum, urine, EDTA plasma, boiled serum or Tris-HCl buffer, pH 7.4, containing CaCl2 for up to 48 hours. Samples were withdrawn at regular intervals, boiled and later analyzed for irTAP using the 9801 antibody-based radioimmunoassay.
DISCUSSION
This paper describes our efforts to develop a radioimmunoassay for the trypsinogen activation peptide TAP. We followed the instructions of Hurley et al. [12] and immunized rabbits with the N-terminally conjugated synthetic octapeptide APFD4K. However, we did not purify our antibody by affinity chromatography on an immobilized YD4K peptide, as suggested by these authors [12]. In this study, we have compared the results obtained with our assay with the results obtained using the commercially available Biotrin TAP ELISA assay.
Our antibody may show some cross-reaction with the proenzyme trypsinogen, which may be an explanation for the minor peak of “high molecular weight” immunoreactivity seen after gel chromatography of unboiled urine (Figure 5). Boiling takes care of this problem, which is also evident from the low amounts of immunoreactivity found in boiled pancreatic juice (Figure 3).
It is clear that the antisera appear to recognize different parts of the activation peptide. The Biotrin antibody reacts in a similar way with both the octapeptide APFD4K (which was used as the standard in our assay) and the pentapeptide D4K (which is used as the standard in the Biotrin assay). In contrast, our assay showed a much lower reactivity against the pentapeptide. In our assay, the immunoreactivity of this peptide was only 2- 4% of the immunoreactivity of the octapeptide (Figure 1). Our conclusion is that our antibody recognizes the middle or Nterminal part of the activation peptide and that the Biotrin antibody recognizes antigenic determinants which are mainly located in the C-terminal part of the octapeptide.
The real aim for the immunization procedure described by Hurley et al. was to obtain an antiserum specific for the C-terminal end of the activation peptide using the N-terminally conjugated pentapeptide and the procedure they used was very successful. We used the N-terminally conjugated octapeptide instead of the pentapeptide and, unfortunately our antibody mainly recognizes the N-terminal part of the activation peptide. This also explains the cross-reaction of our antibody between the activation peptide and the proenzyme.
The differences in immunoreactivity between our polyclonal rabbit antibody (9801) and the Biotrin polyclonal antibody can be used to study the molecular structure of the activation peptide in urine from patients with acute pancreatitis. Most urine samples from patients with acute pancreatitis do not possess any immunoreactivity in our assay, and this must mean that they do not contain the octapeptide at higher concentrations than 1 nmol/L. Only one sample showed increased immunoreactivity. According to gel chromatography, the protein species responsible for this immunoreactivity had the same molecular size as the intact activation peptide (Figure 5). In contrast to the results of our assay, the Biotrin assay showed substantial TAP-like immunoreactivity in several urine samples and, in one sample, a concentration exceeding 200 nmol/L was measured. This discrepancy can be explained by the assumption that urine from patients with acute pancreatitis contains the pentapeptide but not the octapeptide. If this were the case, an obvious explanation would be that the octapeptide is, to a large extent, degraded by N-terminal proteolysis in the circulation before, during or after excretion into the urine.
A characterization of the TAP-like immunoreactivity measured by the Biotrin assay has never been published. The results of this study show that it is composed of material of a molecular size compatible with that of the pentapeptide or the octapeptide (Figure 6). Gel chromatography on a G-50 column is far from the optimal method for showing differences in sizes between the octapeptide and the pentapeptide. This column was chosen to differentiate trypsinogen (molecular weight 23 kDa) from the octapeptide (molecular weight 0.9 kDa). Further studies are required to show the precise nature of the TAP-like immunoreactivity measured in the Biotrin assay.
It appears that the octapeptide is not very stable after the activation of pancreatic juice, as can be seen from Figure 3. Most of the immunoreactivity is destroyed within hours. The results shown in Figure 3 are in accordance with the idea of rapid N-terminal degradation of the octapeptide to the more stable pentapeptide-like molecule, since the reduction in immunoreactivity measured with the Biotrin assay is substantially slower. The octapeptide is probably very unstable in the circulation, since it is rapidly destroyed in the in vitro serum. This degradation can be inhibited by boiling or by chelating agents such as EDTA. Thus, a metalloprotease is probably involved in this degradation.
We have developed an assay with high specificity for the intact activation peptide from human trypsinogen (APFD4K) but our conclusion is that it has very little crossreaction with the pentapeptide D4K, which represents a secondary and major degradation product of the native activation peptide. Using our radioimmunoassay together with the Biotin assay, we found that urine from patients with acute pancreatitis most often contains small amounts of the octapeptide, but substantial amounts of the pentapeptide. One interpretation is that the octapeptide is rapidly degraded in active pancreatic juice and in serum to the more stable pentapeptide molecule. Due to this rapid degradation, antibodies recognizing only the N-terminal part of the activation peptide from trypsinogen are not suitable for monitoring trypsinogen activation in urine in cases of human acute pancreatitis. The pentapeptide D4K is more stable in urine after sampling, but substantial amounts may be degraded before sampling in the circulation during passage to the kidneys.
Despite these drawbacks, our assay can be used for molecular studies of the trypsinogen activation process in in vitro studies where boiling immediately after sampling can stop the reaction and degradation.
This study was supported by grants from the Swedish Medical Research Council projects no 17-X-8305-09A, the Foundations for Research at the University Hospital in Malmö and the Einar and Inga Nilsson Foundation for Surgical Research at the Department of Surgery in Malmö. The funding sources had no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.