Keywords
API, Mobile phase, Rt-HPLC, Covalent bond, Acidic hydrolysis,
Alkaline hydrolysis, Hydrogen bonding, Ionic interactions, Van der Waals
interactions, π-interactions.
Introduction
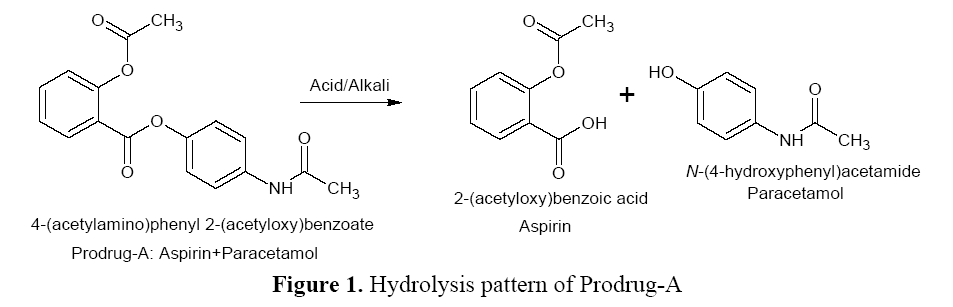
Hydrolysis Pattern of Prodrug-A
Ester linkage of Prodrug–A is ruptured by acid/
alkali to free API (Aspirin and Paracetamol) by
hydrolytic cleavage (Figure 1).
Figure 1: Hydrolysis pattern of Prodrug-A
Esters and amides are two linkages which are
hydrolysable to produce two separete entities.
Esters produce carboxylic acid moiety (–COOH)
and alcohol/phenol moiety (–OH) whereas
amides produce carboxylic acid (–COOH) and
amino moiety (–NH2). Nitriles or cyanides (–
CN) are also hydrolysable group but this is not
a linkage but this is a functional group attached
at the end terminal which is hydrolysed into
carboxylic acid (–COOH) and free amino group
(–NH2). Hydrolysis is possible in acidic and
alkaline medium which ruptures the linkages to
produce the desired moiety.
Our prodrug is formed by covalent bonding
between two APIs to produce Prodrug-A made
by aspirin and paracetamol which produce two
ester and one amide linkage whereas Prodrug-B
is made by indomethacin and paracetamol which
produce one ester and two amide linkages [1,2].
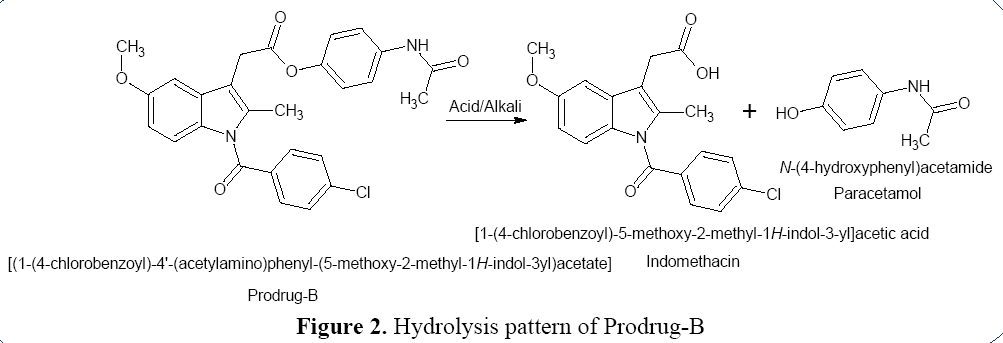
Hydrolysis Pattern of Prodrug-B
Ester linkage of Prodrug-B is ruptured by
acid/alkali to free API (Indomethacin and
Paracetamol) by hydrolytic cleavage (Figure 2).
Figure 2: Hydrolysis pattern of Prodrug-B
Actually acyl [–O–CO–] and ester [–CO–O–]
both have same linkages so Prodrug-A have
two ester and one amide linkages so logP=2.15
whereas Prodrug-B has one ester [–CO–O–] and
two amide [–CO–NH–] linkages so logP=3.94,
because the ester and amide both are susceptible
for hydrolysis but in amide (–CONH–) three
lone pairs of electrons are present (two for
oxygen and one for nitrogen) and in ester (–
COO–) four lone pairs of electrons are present
(four for two oxygen). So the electron density of
ester is greater than amide and electronegativity
of oxygen is 3.44 and for nitrogen is 3.04. So
total electronegativity of ester (–COO–) is
3.44+3.44=6.88 and for amide (–CONH–) is
3.44+3.04=6.44. Hence ester is more susceptible
for hydrolysis compared to amide.
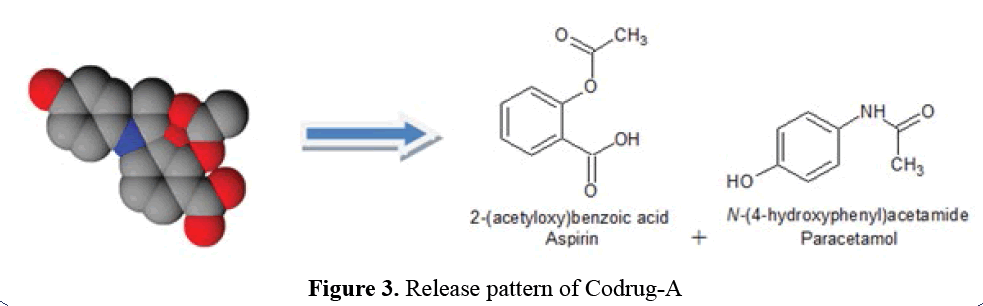
Release Pattern of Codrug-A
Non-covalent interactions such as hydrogen
bonding, ionic interactions, Van der Waals
interaction and π-interaction are ruptured to release free API (Aspirin and Paracetamol)
during hydrolytic cleavage (Figure 3).
Figure 3: Release pattern of Codrug-A
Lone pair profile: Ester>Amide and
Electronegativity profile: Ester>Amide. In
this case Prodrug-A: two ester and one amide,
so lone pair profile: 4×2=6 for ester and 3 for
amide; hence 6 (ester electronegativity)>3(amide
electronegativity) for Prodrug-A (ester is
greater than amide so logP is 2.15). In case of
Prodrug-B: one ester and two amide, so lone pair:
4 for ester and 3×2=6 for amide; hence 6 (amide
electronegativity)>4 (ester electronegativity) for
Prodrug-B (Amide is greater than ester so logP
is 3.94). Hence Prodrug-B is more nonpolar than
Prodrug-A [3,4].
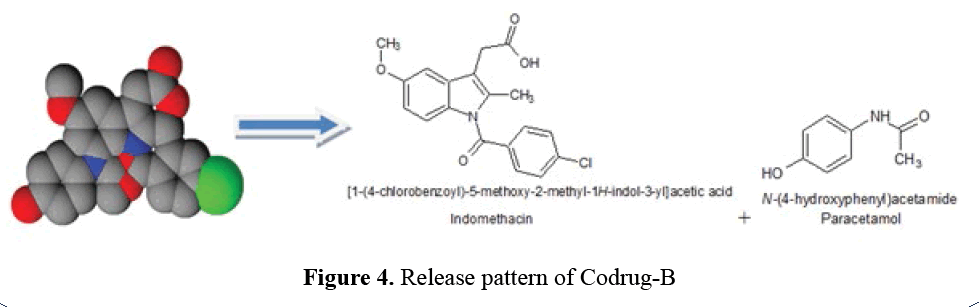
Release Pattern of Codrug-B
Non-covalent interactions such as hydrogen
bonding, ionic interactions, Van der Waals
interactions and π-interactions are ruptured to
release free API (Indomethacin and Paracetamol)
during hydrolytic cleavage (Figure 4).
Figure 4: Release pattern of Codrug-B
Prodrug-A and Prodrug-B both have ester
linkages which is ruptured in acidic and alkaline
pH into two different moieties. Prodrug-A
produces aspirin and paracetamol and Prodrug-B
produces indomethacin and paracetamol. These
two products produce two peaks at HPLC to show
different Rt values according to their polarity
followed by logP: Aspirin (1.19), Indomethacin
(3.10) and Paracetamol (0.34). Since the prodrugs
are made by covalent linkages of esters and
amides so it takes some time to be ruptured into
desired API according to the polarity whereas in
Codrug-A and Codrug-B which are made by noncovalent
interactions such as hydrogen bonding,
ionic interactions, Van der Waals interactions
and π-interactions between two APIs, so the
release of parent molecule is faster than prodrug
both in acidic as well as in alkaline pH because
prodrug is made by covalent bonding between
two APIs [5-7].
Selection of Ratio of Mobile Phase
The solution containing 100 μg/ml of Prodrug-A,
Prodrug-B, Codrug-A and Codrug-B respetively
was chromatographed with mobile phase of
different ratio of methanol and water.
Experimental
Reagents and Materials, Prodrug-A synthesized
in our college lab, Methanol (HPLC grade,
Finar Chemicals Ltd, Ahmedabad, India), Water (HPLC grade, Finar Chemicals Ltd, Ahmedabad,
India).
Equipments and Instruments
Shimadzu HPLC instrument (LC-2010 CHT)
equipped with prominence diode array detector
(SPD-M20A) (Software LC Solution), Analytical
balance (Acculab ALC-2014, Huntingdon Valley,
PA), Ultra sonicator (EN 30 US, Enertech Fast
Clean, Mumbai, India), Hot air oven (TO-90S,
Thermolab, Mumbai, India), pH meter (Thermo
Electron Corp., Pune, India) (Table 1).
Table 1. Selection of mobile phase for Prodrug-A, Prodrug-B, Codrug-A and Codrug-B
| Prodrug-A/ Codrug-A |
Trials |
Ratio |
Remark |
Prodrug-B/ Codrug-B |
Trials |
Ratio |
Remark |
| 1 |
Methanol:
Water (60:40) |
Tailing |
1 |
ACN: Water
(80:20) |
Tailing |
| 2 |
Methanol: Water (70:30) |
Tailing |
2 |
ACN: Water
(70:30) |
Tailing |
| 3 |
ACN: Water
(60:40) |
Tailing |
3 |
ACN: Methanol
(80:20) |
Tailing |
| 4 |
ACN: Water
(70:30) |
Tailing |
4 |
ACN: Methanol
(70:30) |
Tailing |
| 5 |
Methanol: Water
(80:20) |
Symmetrical
peak |
5 |
Methanol: Water
(80:20) |
Tailing |
| |
6 |
Methanol: Water
(70:30) |
Symmetrical peak |
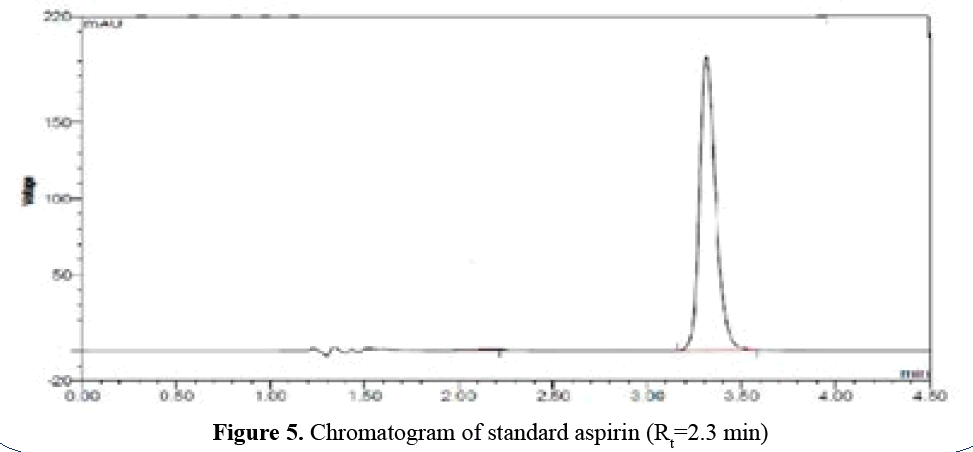
A. Aspirin profile: Standard aspirin at 284 nm in
mobile phase ACN: Triethylamine buffer (50:50
v/v):-(logP=1.19). Aspirin releases moderately
slow (Rt=2.3 min) because it’s logP is 1.19
(semipolar) (Figure 5).
Figure 5: Chromatogram of standard aspirin (Rt=2.3 min)
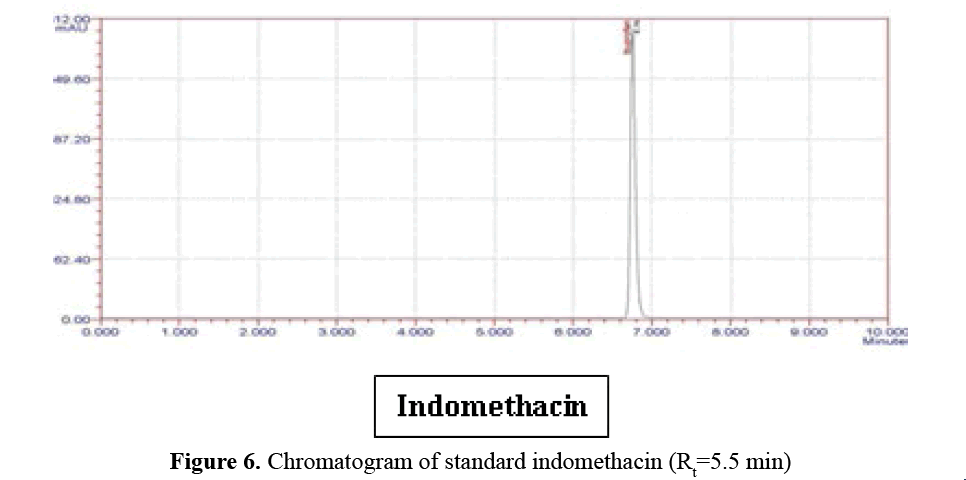
B. Indomethacin profile: Standard Indomethacin
peak at 278 nm in mobile phase Water: ACN
(80:20 v/v):- (logP=3.10). Indomethacin releases
slow (Rt=5.5 min) because it’s logP is 3.97
(nonpolar) (Figure 6).
Figure 6: Chromatogram of standard indomethacin (Rt=5.5 min)
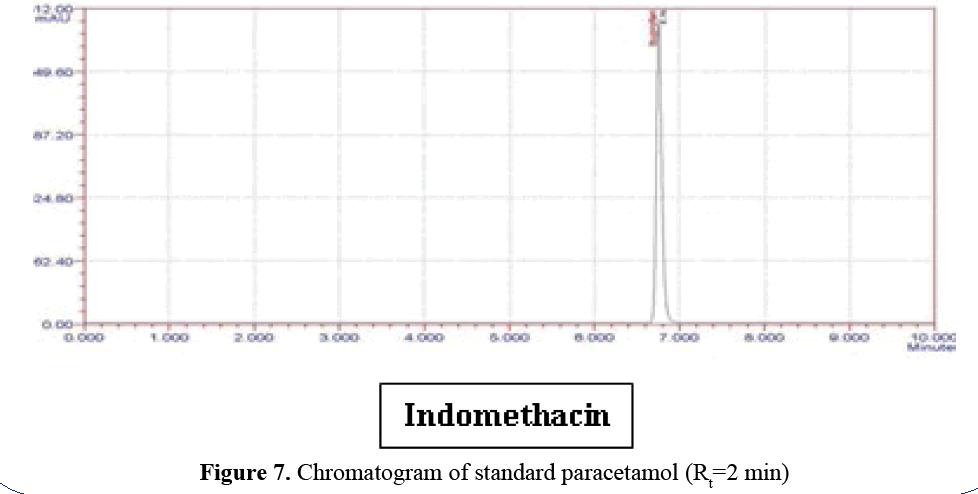
C. Paracetamol profile: Standard paracetamol
peak at 230 nm in mobile phase. Phosphate
Buffer: ACN (40:60 v/v):- (logP=0.34).
Paracetamol releases first (Rt=2 min) because it’s
logP is 0.34 (highly polar) (Figure 7).
Figure 7: Chromatogram of standard paracetamol (Rt=2 min)
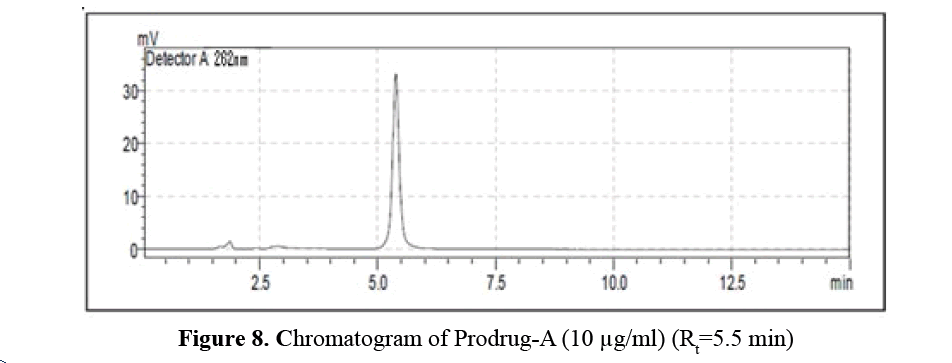
Prodrug-A Profile (logP=2.15)
Prodrug-A has logP 2.15 so it releases slowly
(Rt=5.5 min) due to nonpolar nature (Figure 8).
Figure 8: Chromatogram of Prodrug-A (10 μg/ml) (Rt=5.5 min)
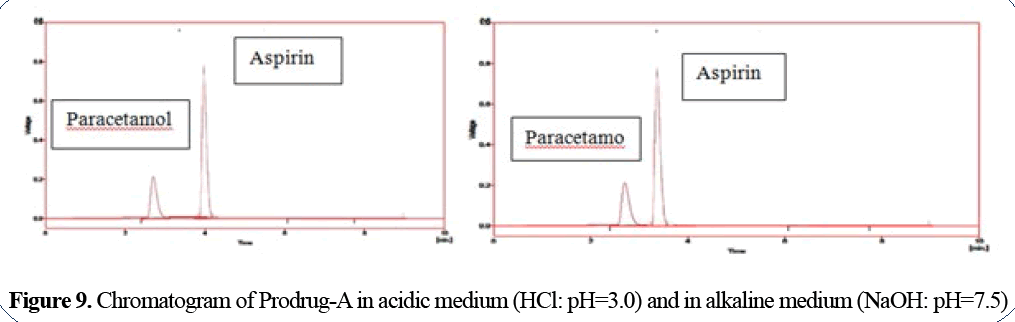
Paracetamol has Rt=2.75 min in acidic medium
and 2.8 min in alkaline medium; aspirin has Rt=4
min in acidic medium and 3.8min in alkaline
medium (Figure 9).
Figure 9: Chromatogram of Prodrug-A in acidic medium (HCl: pH=3.0) and in alkaline medium (NaOH: pH=7.5)
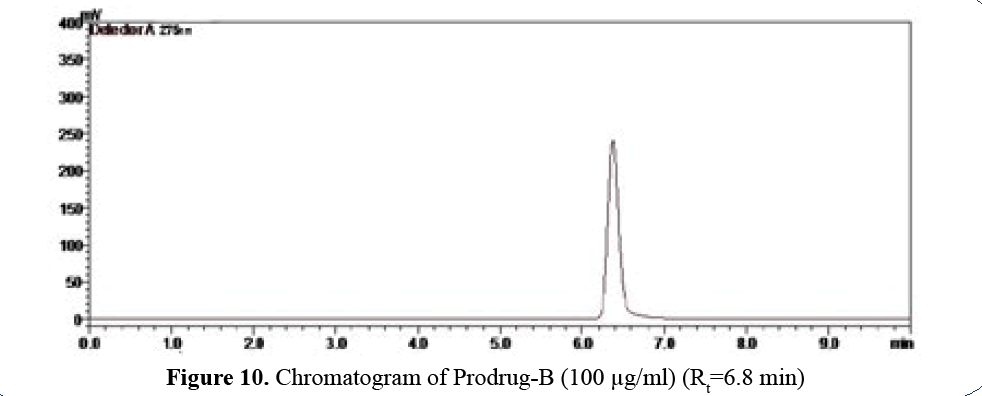
Prodrug-B Profile (logP=3.94)
Prodrug–B has logP 3.94 and Rt=6.8 min so
release rate is low (Figure 10).
Figure 10: Chromatogram of Prodrug-B (100 μg/ml) (Rt=6.8 min)
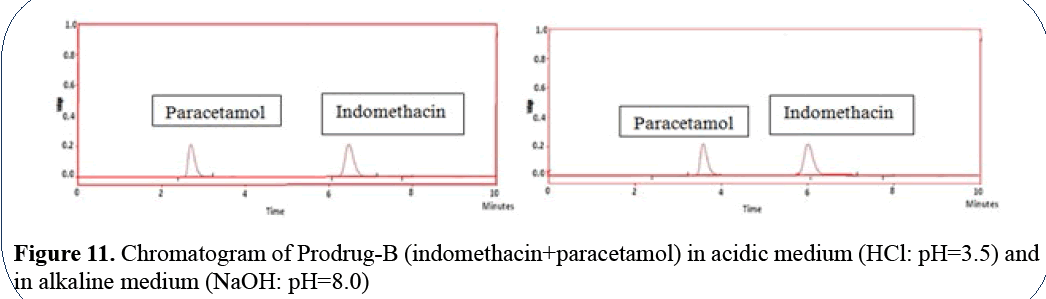
Paracetamol has Rt=2.5 min in acidic medium
and 3.5 min in alkaline medium; indomethacin
has Rt=6.35 min in acidic medium and 6.15 min
in alkaline medium (Figure 11).
Figure 11: Chromatogram of Prodrug-B (indomethacin+paracetamol) in acidic medium (HCl: pH=3.5) and
in alkaline medium (NaOH: pH=8.0)
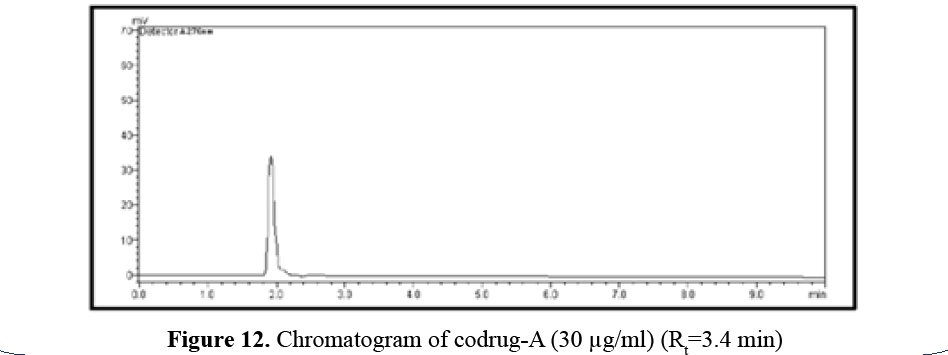
Codrug-A Profile
Codrug-A has logP 1.55 so it releases fast due
to polar nature. Codrug-A has logP 1.55 so it is
polar in nature so it shows Rt=3.4 mins (Figure
12).
Figure 12: Chromatogram of codrug-A (30 μg/ml) (Rt=3.4 min)
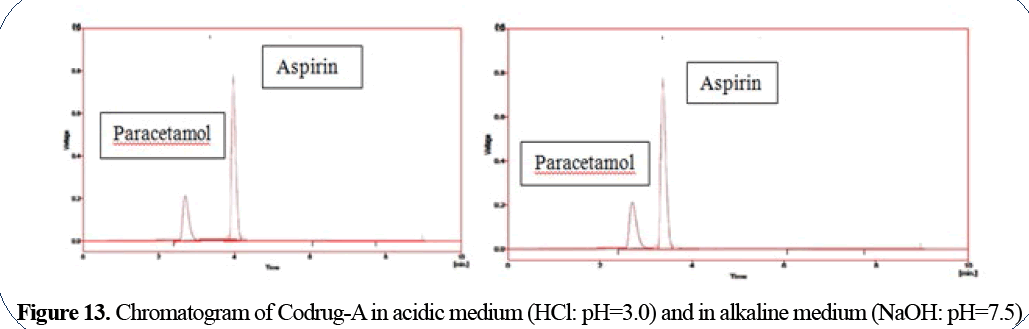
Paracetamol has Rt=2.75 min in acidic medium
and 2.8 min in alkaline medium; aspirin has Rt=4 min in acidic medium and 3.8 min in alkaline
medium (Figure 13).
Figure 13: Chromatogram of Codrug-A in acidic medium (HCl: pH=3.0) and in alkaline medium (NaOH: pH=7.5)
Codrug-B Profile
Codrug-B has logP 3.42 so it releases slow due
to nonpolar nature.
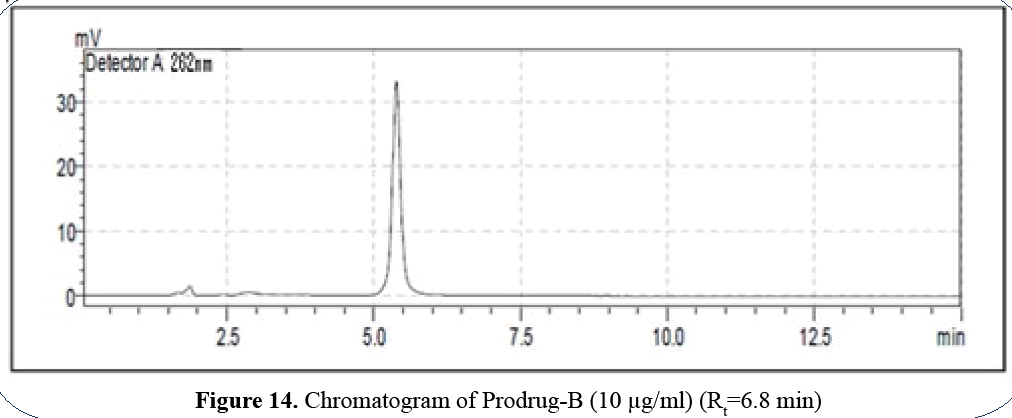
Prodrug-B is highly nonpolar so it releases at Rt 6.8 min (Figures 14 and 15).
Figure 14: Chromatogram of Prodrug-B (10 μg/ml) (Rt=6.8 min)
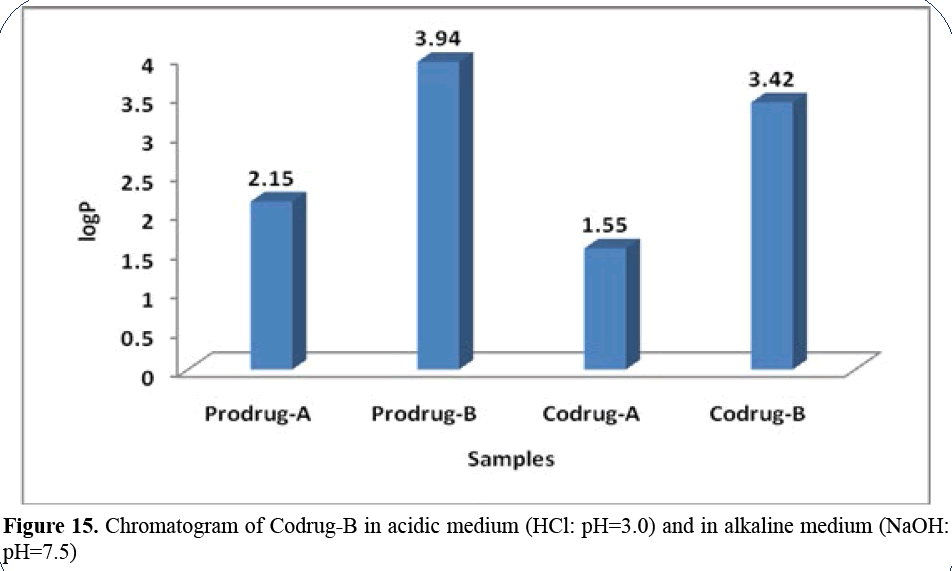
Figure 15: Chromatogram of Codrug-B in acidic medium (HCl: pH=3.0) and in alkaline medium (NaOH:
pH=7.5)
Paracetamol has Rt=3.4 min in acidic medium
and 3.4min in alkaline medium; indomethacin
has Rt=6.8 min in acidic medium 6.35 min in
alkaline medium (Figures 16 and 17).
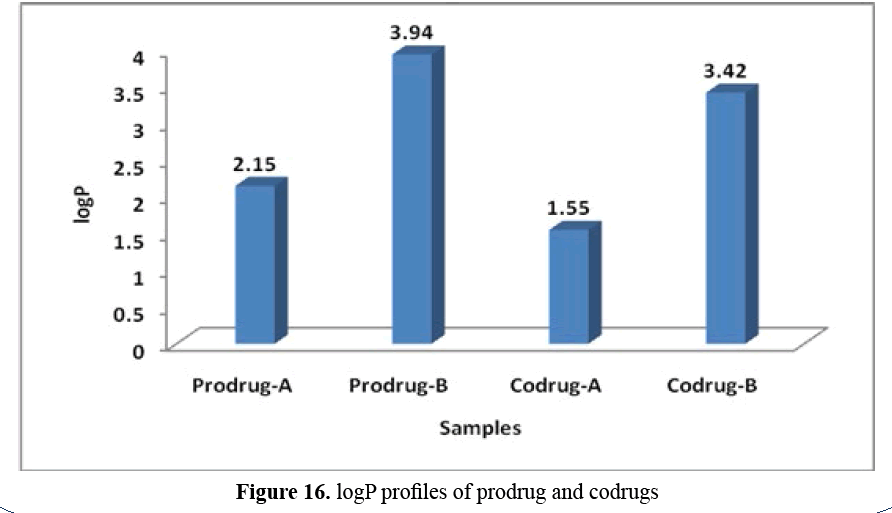
Figure 16: logP profiles of prodrug and codrugs
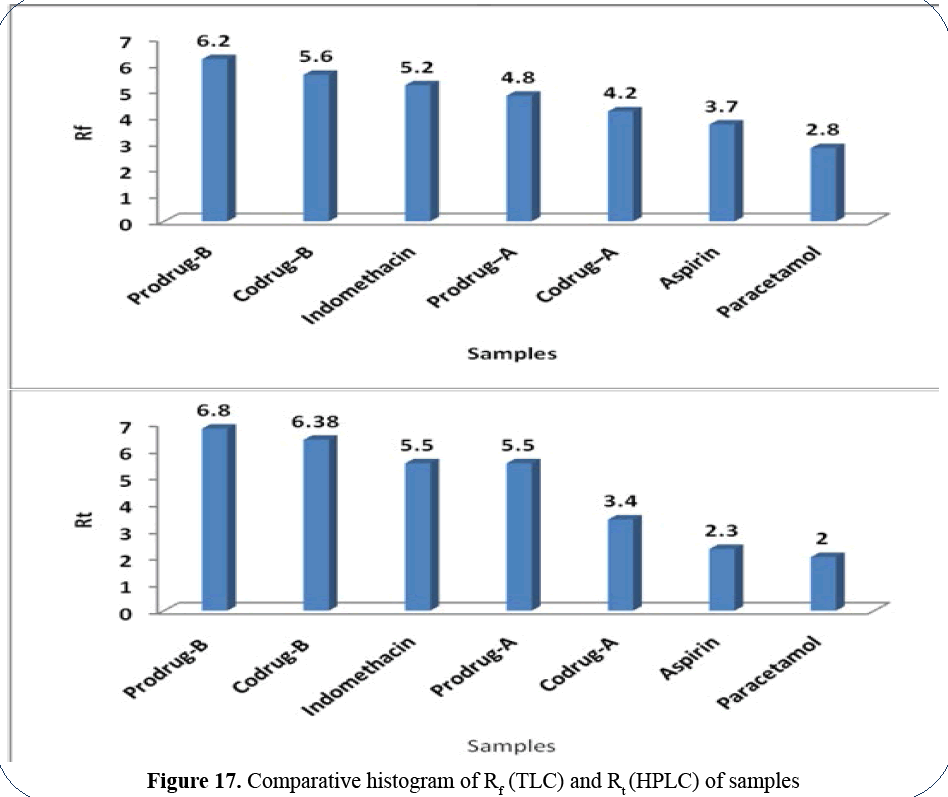
Figure 17: Comparative histogram of Rf (TLC) and Rt (HPLC) of samples
Conclusion
API Profile
Aspirin releases moderately slow (Rt=2.3
min) because it’s logP is 1.19 (semipolar).
Indomethacin releases slow (Rt=5.5 min)
because it’s logP is 3.10 (nonpolar). Paracetamol
releases first (Rt=2 min) because it’s logP is 0.34
(highly polar).
Prodrug Profile
Prodrug-A has logP 2.15 so it releases slowly
due to nonpolar nature. Prodrug-A has Rt=5.5
min. Paracetamol has Rt=2.75 min in acidic
medium and 2.8 min in alkaline medium; Aspirin
has Rt=4 min in acidic medium and 3.8 min in
alkaline medium. Prodrug-B has Rt 6.8 min.
Prodrug-B has logP 3.94 so release rate is slower.
Paracetamol has Rt=2.5 min in acidic medium
and 3.5 min in alkaline medium; Indomethacin
has Rt=6.35 min in acidic medium and 6.15 min
in alkaline medium.
Codrug Profile
Codrug-A has Rt=3.4 min and logP 1.55.
Paracetamol has Rt=2.75 min in acidic medium
and 2.8 min in alkaline medium; Aspirin has Rt=4
min in acidic medium and 3.8 min in alkaline
medium. Codrug-B has logP 3.42. Paracetamol
has Rt=3.4 min in acidic medium and 3.4 min in
alkaline medium; Indomethacin has Rt=6.8 min
in acidic medium 6.35 min in alkaline medium.
logP profile: Prodrug-B (3.94)>Codrug-B
(3.42)>Prodrug-A (2.15)>Codrug-A (1.55).
Rf profile: Prodrug-B (6.2)>Codrug-B
(5.6)>Indomethacin (5.2)>Prodrug-A
(4.8)>Codrug-A (4.2)>Aspirin
(3.7)>Paracetamol (2.8).
Rt profile: Prodrug-B (6.8 min)>Codrug-B
(6.38 min)>Indomethacin (5.5 min)>Prodrug-A (5.5 min)>Codrug-A (3.4 min)>Aspirin (2.3
min)>Paracetamol (2 min).
Acknowledgement
The authors Debojyoti Basu and Divyesh
Sharma both students of B.Pharm-V at Shri
Sarvajanik Pharmacy College, Mehsana did
this project under the esteemed guidance of
Prof. Dr. Dhrubo Jyoti Sen. This project is
fully based on basic chemical science including
physical synthesis (codrug) as well as chemical
synthesis (prodrug) and instrumental analysis
through elemental analysis, chromatography
of drug followed by API, CHN%, Prodrug,
Codrug, Co–crystallization, logP, TLC, UV λmax,
IR Spectra, Mass Spectra, Hydrogen bonding,
Ionic interactions, Van der Waals interactions,
π–interactions.
Chromatographic release profile of active
pharmaceutical ingredients of synthesized
prodrug and codrug of aspirin+paracetamol and
indomethacin+paracetamol in physiological
fluids is the outcome of the total efforts and
applications of B.Pharm. course contents into
practical approach. The authors are thankful to
Shri Sarvajanik Pharmacy College, Mehsana
for providing drugs and laboratory facilities to
perform synthesis of prodrug and codrug with
their analytical profiles to fulfill this project
with grand success. The authors are thankful
to quality assurance lab of Shri Sarvajanik
Pharmacy College, Mehsana for TLC and HPLC
studies respectively.
References
- Patel JG, Sen DJ. Synthesis of Prodrug of ester and amide linkages of NSAID having carboxylic acid, phenolic and imino groups. World J Pharm Pharm Sci. 2016;5(11):897-908.
- Sen DJ, Patel JG. Logarithmic partition coefficient comparison study and molecular weight of synthesized Prodrugs of ibuprofen+paracetamol, diclofenac sodium+paracetamol and ibuprofen+diclofenac sodium. Am J Drug Deliv. 2016;4(5):64-8.
- Sen DJ. Correlation approach of in–vivo and in-vitro hydrolytic metabolism of ester linkage of prodrug made of indomethacin and paracetamol in RP-HPLC in acidic and alkaline medium: European Journal of Biomedical and Pharmaceutical Science. 2017;4(7):424-33.
- Sen DJ. RP-HPLC study of in-vitro biotransformation of prodrugs of ester and amide linkages of ibuprofen, diclofenac sodium and paracetamol in acidic and alkaline medium. Pharma Tutor. 2017;5(8):49-65.
- Gediya PA, Sen DJ. Co-crystallization technology: A magic bullet in medicinal chemistry. International Journal of Advances in Pharmaceutical Research. 2013;4(8):2071-76.
- Akhani P, Thakkar A, Shah H, et al. Correlation approach of prodrug and codrug in biotransformation. European Journal of Pharmaceutical and Medical Research. 2017;4(5):488-500.
- Basu D, Sharma D, Sen DJ. Comparative physicochemical correlation study of synthesized prodrug and codrug of aspirin+paracetamol and indomethacin+paracetamol by covalent and non-covalent bonding. World J Pharm Res. 2017;6(8):2066-83.