Keywords
Acute type A aortic dissection; Prolonged mechanical ventilation;
Malperfusion; Limb ischemia; COPD.
Introduction
Acute type A aortic dissection (AAAD) remains one of the most
life-threatening cardiovascular disorders with high morbidity
and mortality. Past reports regarding clinical outcomes of
surgically treated AAAD revealed that in-hospital mortality
ranged from 22-58% [1-4]. Under such critical conditions,
prolonged mechanical ventilation (PMV) was necessary for
some patients after surgery.
Compared to other cardiovascular surgical procedures, patients
complicated with AAAD can suffer from hemodynamic instability
on admission, mal-perfusion to vital organs, prolonged CPB
time which can lead to low cardiac output syndrome (LOS) and
uncontrollable bleeding during or after surgery, and neurological
disorders [2]. All of these factors can create the need for PMV
after AAAD repair (AAADR). In addition, systemic inflammatory
response-related lung injuries caused by the acute aortic dissection itself can accelerate PMV following AAADR [5-7].
Although there are several reports focusing on the contributing
factors of PMV after cardiac surgery and the monetary costs on
their clinical outcomes [8-17], little evidence is known regarding
the contributing factors for PMV following AAADR. The aim of
this study was to clarify influencing factors for PMV following
AAADR on clinical outcomes.
Methods
The primary entry tear was routinely resected. In the case where an intimal tear was located at the ascending aorta, replacement
of the ascending aorta was performed. Also, when there was an
intimal tear in the aortic root or the aortic root was dilated, the aortic root was replaced. If no initial tear was identified at the ascending
aorta, Total Arch Replacement (TAR) with the elephant
trunk technique was performed. Under median sternotomy in
the supine position, CPB was initially established through the right axillary artery and femoral arteries for arterial cannulations and bicaval
cannulations for venous drainage. In the case where the right axillary artery was not available due to its dissection, the isolated unilateral
femoral artery was cannulated. A left
ventricular vent tube was inserted through the right superior
pulmonary vein. After bladder temperature decreased to 25°C,
the ascending aorta was opened under circulatory arrest. Then,
Antegrade Selective Cerebral Perfusion (ASCP) was initiated by clamping the brachiocephalic artery and inserting a 12F balloon tipped
cannulas into the left common carotid and left subclavian artery, respectively. Myocardial protection was achieved with retrograde delivery
of cold crystalloid solution. The aortic segment, including the intimal tear, was resected. When performing TAR, a sealed 4-branched graft (J
graft SHIELD NEO., Japan Lifeline Co. Ltd., Shinagawa, Tokyo, Japan) was used. An elephant trunk graft was inserted into the distal aorta.
The distal aortic stump was reinforced with felt strips followed by open distal anastomosis with continuous 4-0 monofilament sutures.
Circulation of the lower body was restarted through the side branch of the branched graft after completion of open distal anastomosis.
The distal aortic stump was reinforced with felt strips followed by open distal anastomosis with continuous 4-0 monofilament sutures.
Circulation of the lower body was restarted through the side branch of the branched graft after completion of open distal anastomosis.
Systemic rewarming was initiated followed by the resection of the proximal aorta at 10 mm distal to the sino-tubular junction and
reinforcement of the aortic stump was achieved with a pair of Teflon felt strips, both inside or outside of the aortic wall. Proximal
anastomosis was performed with continuous 4-0 monofilament sutures. Finally, the left subclavian artery, the left common carotid artery,
and the brachiocephalic artery were
reconstructed step-by-step. On the other hand, in performing the replacement of the ascending aorta, a sealed single branched
graft (J graft, Japan Lifeline, Tokyo) was used. Distal and proximal anastomosis was performed in a similar fashion as that of TAR under
moderate hypothermic circulatory arrest with ASCP.
Respirator discontinuation protocol
After surgery, mechanical ventilation in volume-control mode on the Servo-I (Maquet Critical Care, Solna, Sweden) at an appropriate
inspired oxygen fraction (FiO2) was started at the time of being transferred to the Intensive Care Unit (ICU) to keep arterial oxygen
saturation (SaO2) above 97%. Once hemodynamic stability without excessive bleeding, normothermia, normal
acid-base balance, full recovery from anesthesia, and muscle
relaxation were obtained, the discontinuation process
was initiated in the synchronized intermittent mandatory ventilation plus the pressure support mode. The trachea was
extubated when the patient’s breathing was comfortable with a tidal volume >6 ml/kg, respiratory rate <25/min, vital capacity >15 ml/kg,
arterial oxygen tension (PaO2/FiO2) >200, and arterial carbon dioxide tension (PaCO2) <50 mmHg under positive endo-expiratory pressure
(PEEP) of 3-5 cm H2O without any signs of excessive pharyngeal and tracheal secretion. If the patient did not meet all of the discontinuation
criteria or the extubation criteria, the process was suspended,
and appropriate treatments under respirator support were continuously performed.
Statistical analysis
Continuous data are presented as median and interquartile
range. Normally distributed data were analyzed using 2-tailed t-tests, and non-normally distributed data were compared with the Mann-
Whitney test, as appropriate. Categorical variables
are given as a count and percentage of patients and compared using the χ2 test. When any expected frequency was less than 1, or 20% of
expected frequencies were less than or equal to 5, Fisher’s exact test was used. Clinical outcomes between both groups and the influencing
factors of PMV were analyzed by multivariate analysis of variance (MANOVA). A p-value of <0.05 was considered significant. All data were
analyzed using the Statistical Analysis Systems software JMP 12.0 (SAS Institute Inc.,
Cary, NC, USA).
Patients received emergency surgery under the diagnosis of AAAD after written informed consent was obtained from all patients or
their families. In proceeding with this study, approval from the
Institutional Review Board was granted and for reporting patient information.
Results
Preoperative backgrounds (Table 1), demonstrate the percentages of those with chronic obstructive pulmonary disease (COPD) (13% vs.
3%, p=0.002), redo operative cases (8% vs. 1%, p=0.006), mal-perfusion to heart (11% vs. 1%, p<0.001) or lower limbs (20% vs. 8%,
p=0.004) were significantly higher in Group B than in Group A. Procedure related data in Table 2A revealed that operation time (OT) (485 ±
140 vs. 387 ± 97 min, p<0.001), CPB time (251 ± 78 vs. 212 ± 57 min, p<0.001), aortic cross clamp (ACC) time (156 ± 52 min vs. 132 ± 36 min,
p<0.001) , ventilation time (183.9 ± 129.0 h vs. 29.6 ± 22.2 h, p<0.01 ), and postoperative ICU stay (34 ± 20 vs. 27 ± 16 days, p<0.001) were
significantly longer in group B than in group A. There were no significant differences in re-incubation rate within 24 h after extubation (2% vs. 2.6%, p=0.87) between both groups. There was more intraoperative bleeding amount (IBA) (3128 ± 1852 vs. 2093 ± 1314 ml,
p<0.001) identified in Group B. The percentage
of cases complicated with postoperative acute renal failure
(ARF) (17% vs. 1%, p<0.001) were significantly higher in Group
B. The reasons for PMV are demonstrated in Table 2B. The 30-day mortality was significantly higher in Group B than in
Group A (23% vs. 5%, p<0.001). Multivariate analysis in Table 3 demonstrates that COPD, preoperative mal-perfusion to vital organs or lower limbs, and operation time were significant influencing factors
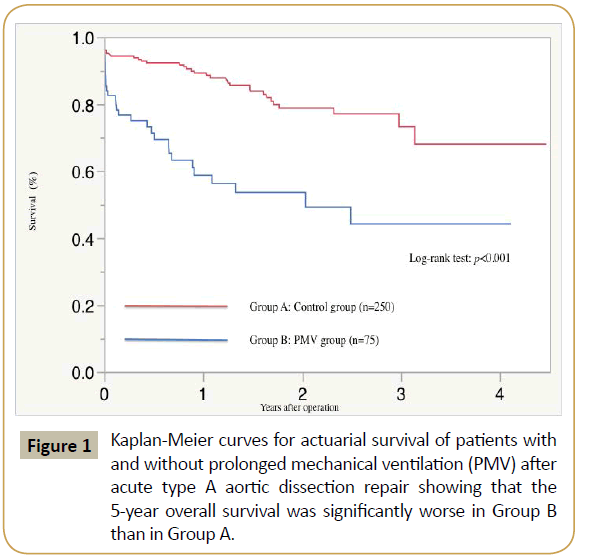
for PMV. Kaplan-Meier survival curve is shown in Figure 1. The 5-year overall survival (44.2% vs. 68%, Log-rank test; p<0.001) was
significantly worse in Group B
than in Group A in this study.
|
Group A
MV ≤ 72 h (n=250) |
Group B
MV>72 h (n=75) |
p value |
| Age, mean (SD), years |
69 ± 13 |
70 ± 12 |
0.5 |
| Elderly (aged > 75 years) (%) |
99 (40) |
32 (43) |
0.69 |
| Male gender (%) |
114 (46) |
40 (75) |
0.29 |
| BSA, mean (SD), m2 |
1.6 ± 0.2 |
1.6 ± 0.2 |
0.4 |
| ˙Hypertension (%) |
179 (72) |
59 (79) |
0,24 |
| Hyperlipidemia (%) |
61 (24) |
23 (31) |
0.29 |
| Diabetes (%) |
9 (19) |
3 (4) |
0.43 |
| Past or currenet smoking (%) |
90 (37) |
23 (31) |
0.41 |
| COPD (%) |
8 (3) |
10 (13) |
0.002 |
| Serum creatinine, mean (SD), mg/dl |
1.2 ± 1.7 |
1.3 ± 0.8 |
0.64 |
| Chronic hemodialysis (%) |
7 (3) |
2 (3) |
1 |
| History of CVD(%) |
24 (10) |
7 (9) |
1 |
| Histpry of IHD (%) |
6 (2.5) |
5 (7.5) |
0.31 |
| Previous cardiac surgery (%) |
3 (1) |
6 (8) |
0.006 |
| Marfan sydrome (%) |
29 (12) |
16 (21) |
0.037 |
| * Malperfusion status |
| Brain |
29 (12) |
16 (21) |
0.037 |
| Heart |
3 (1) |
8 (11) |
<0.001 |
| Spinal cord |
1 (0.4) |
0 (0) |
1 |
| Bowels |
7 (3) |
5 (7) |
0.16 |
| Kidneys |
5 (2) |
4 (5) |
0.22 |
| Lower limbs |
19 (8) |
15 (20) |
0.004 |
Table 1 Comparison between both groups in preoperative patient’s backgrounds.
|
Group A MV ≤ 72h (n=250) |
Group B MV>72 h (n=75) |
p value |
| TAR (%) |
47 (117/250) |
47 (35/75) |
1 |
| OP time, mean (SD), min |
389 ± 97 |
485 ± 140 |
<0.001 |
| CPB time, mean (SD), min |
212 ± 57 |
251 ± 78 |
<0.001 |
| SCP time, mean (SD), min |
71 ± 26 |
69 ± 35 |
0.81 |
| ACC time, mean (SD), min |
132 ± 36 |
156 ± 52 |
<0.001 |
| CA time, mean (SD), min |
52 ± 14 |
54 ± 14 |
0,26 |
| IBA, mean (SD), ml |
2093 ± 1314 |
3128 ± 1852 |
<0.001 |
| BT, mean (SD), units |
6.3 ± 4.8 |
8.3 ± 6.2 |
0.004 |
| Ventilation Time (h) |
29.6 ± 22.2 |
183.9 ± 129.0 |
<0.01 |
| ICU stay, mean (SD), days |
3.5 ± 2.1 |
10.0 ± 7.0 |
<0.001 |
| Hospital stay, mean (SD), days |
27 ± 16 |
34 ± 20 |
0.004 |
| Renal failure (Serum Creatine >2.0mg/dI) (%) |
3 (1) |
13 (17) |
<0.001 |
| CVA (%) |
2 (1) |
5 (7) |
0.008 |
| Re-incubation within 24 h (%) |
5 (2) |
2 (2.6) |
0.87 |
| Traceheostomy (%) |
2 (1) |
5 (7) |
0.008 |
| SSI (%) |
0 (0) |
0 (0) |
1 |
| Operative mortality (%) |
12 (5) |
16 (19) |
<0.001 |
| In-hospital mortality (%) |
12 (4.8) |
7 (23) |
<0.001 |
Table 2A Comparison between both groups in procedure related data.
|
Group B MV>72 h (n=75) |
| Acute Kidney Injuries (%) |
24 (32) |
| Respiratory Failure (%) |
22 (29) |
| Cerebro Vascular Accident (%) |
24 (32) |
| Low Cardiac Output Syndrome (%) |
3 (4) |
| Acute Liver Dysfunction (%) |
1 (1.3) |
| Gastrointestinal Perforation (%) |
1 (1.3) |
Table 2B The breakdowns of the primary reasons for prolonged mechanical ventilation.
|
Odds Ratio |
95%CI |
p value |
| COPD |
6.2 |
2.0-19.6 |
0.004 |
| Malperfusion |
2.4 |
1.3-4.4 |
0.009 |
| OP time |
1.04 |
1.02-1.08 |
0.003 |
Table 3 The risk factors for prolonged mechanical ventilation on multivariate analysis.
Figure 1: Kaplan-Meier curves for actuarial survival of patients with and without prolonged mechanical ventilation (PMV) after acute type A aortic dissection repair showing that the 5-year overall survival was significantly worse in Group B than in Group A.
Discussion
There have been several reports of correlations between acute aortic dissection and impaired pulmonary oxygenation [5,18-21]. The previously reported studies mainly referred to patients receiving conservative medical treatments for acute aortic dissection, and exacerbated oxygenation was caused by systemic inflammatory reaction syndrome (SIRS) rather than pulmonary congestion [18-21]. From the viewpoint of pathological findings in the acute dissected aorta, infiltration of macrophages and leukocytes and increased expression of genes related to inflammatory processes, including interleukin-6 and interleukin-8, are frequently found [20,22]. These findings suggested that localized aortic injuries caused by acute aortic dissection have the potential to produce humoral factors that penetrate the pulmonary vasculature and activate both leukocytes and the pulmonary vascular endothelium. Also, Sugano noted that the powerful assault of intimal rupture and the dissection propagation into the media might cause activation of the cellular and humoral inflammatory systems, which eventually led to oxygenation insufficiency. Kimura proposed that this inflammatory related lung injury might well be a major underlying mechanism of respiratory impairment following AAADR [23].
In the present study, 23% of patients who underwent emergency AAADR depended on PMV for more than 72 h. This percentage was significantly higher than the previously reported incidence of PMV following coronary artery bypass grafting (CABG), ranging from 2.6 to 6.5% [8-17]. Advanced age, female sex, obesity, COPD, smoking, history of stroke, acute kidney injury, preoperative shock, preoperative use of IABP, concomitant valve procedures, redo cardiac surgery, prolonged CPB time and ACC time, emergency surgery, and unstable angina were revealed as risk factors for PMV after adult cardiac surgery in the previously reported articles [8-10,12-17]. On the other hand, the risk factors for PMV for more than 48 h following AAADR, preoperative shock, malperfusion to coronary arteries requiring concomitant CABG and lower limbs, and preoperative renal failure were identified [23]. In the present study, COPD, malperfusion to vital organs, including brain, coronary arteries or lower limbs, longer OT, and IBA were shown as independent risk factors for PMV for more than 72 h after AAADR. In patients complicated with coronary ischemia, preoperative hemodynamic instability is quite common, and concomitant procedures, including percutaneous coronary intervention (PCI) prior to the establishment of CPB or CABG are frequently required. These additional treatments can lead to increased OT as well as CPB time [24,25].
As previously shown in the literature by Kimura [23], clinical analysis in the present study demonstrated that limb ischemia was another risk factor for PMV as well. The ratio of AAAD complicated with limb ischemia was 25-33% and was related to increased morbidity and mortality [26]. Although the relationships between limb ischemia and PMV following AAADR have not clearly been identified yet, rhabdomyolysis and acute kidney failure as a consequence of reperfusion injury from prolonged ischemic time to the lower limbs can have the potential to increase the duration of mechanical ventilation [23]. Therefore, we consider comprehensive treatment strategies to recover from malperfusions to visceral organs and lower limbs as promptly as possible during the AAADR are important in improving in-hospital mortality and long-term outcomes in Group B. In this regard, Hsu reported clinical efficacy of complete attachment (PETTICOAT) technique to facilitate distal aortic remodeling, including increase in diameter of true lumen and decrease in that of false lumen at the time of performing AAADR [27]. In treating those complicated with malperfusions to visceral organs and lower limbs, we consider it is imperative to transfer the patients to the operating room immediately after the definitive diagnosis of those disorders on enhanced CT followed by the establishment of CPB, including antegrade or retrograde arterial perfusions through the true lumen as soon as possible to shorten ischemic time of malperfusion status. We consider the PETTICOAT technique can be applied as an effective endovascular treatment in cases of persistent malperfusion status regardless of central repair, including intimal tear resection and elephant trunk insertion or frozen elephant trunk technique [28].
From the viewpoint of management in the intensive care unit (ICU) after surgery, multidisciplinary treatment approaches, which include cardiovascular surgeons, intensive care specialists, ICU-registered nurses, respiratory physical therapists, pharmacists, clinical engineers, registered nutritionists, and medical technologists are expected to promote inter-professional collaboration among the participating members stated above, which can significantly reduce the duration of PMV following AAADR [29,30].
This investigation has some limitations. First, compared to other studies of PMV after cardiovascular surgery, we concentrated solely on those receiving AAADR, yielding a relatively small number of enrolled cases. So, further investigations involving larger sample sizes are required. Secondly, due to the emergency nature of AAADR, reliable information concerning preoperative cardiac functions on echocardiography was not fully obtained. In some cases, there were no choices but to go directly to the operating room without echocardiographic evaluations because of hemodynamic instability. Thirdly, the presence of COPD was judged on only the patient’s medical history and the oral medications prescribed. Therefore, we might have underestimated the precise number of those suffering from COPD preoperatively.
Conclusion
In the present study, 23% of those who underwent emergency AAADR depended on PMV for more than 72 h in the present study. PMV was significantly associated with preoperative malperfusion to vital organs or lower limbs, OT, and IBM. Our study revealed that managing these influencing factors perioperatively can contribute to improving surgical and long-term outcomes of AAADR.
Disclosure Statement
All of the authors have nothing to disclose and also state no conflict of interest in the submission of this manuscript.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on the reasonable request.
References
- Rangachari V, Sundararajan I, Sumathi V, Krishna KK (2006) Laryngeal sequelae following prolonged intubation: a prospective study. Indian J Crit Care Med 10: 171ÃÆÃâÃââÃÆââ¬Å¡Ã¢ââ¬Å¡Ã¬ÃÆââ¬Å¡Ã¢ââ¬Ã
â175.
- Colton House J, Noordzij JP, Murgia B, Langmore S (2011) Laryngeal injury from prolonged intubation: a prospective analysis of contributing factors. Laryngoscope 121: 596ÃÆÃâÃââÃÆââ¬Å¡Ã¢ââ¬Å¡Ã¬ÃÆââ¬Å¡Ã¢ââ¬Ã
â600.
- Matta RI, Halan BK, Sandhu K (2017) Postintubation recurrent laryngeal nerve palsy: A review. J Laryngol Voice 7: 25-28.
- Panda NK, Mann SB, Raja BA, Batra YK, Jindal SK (1998) Fibreoptic assessment of post intubation laryngotracheal injuries. Indian J Chest Dis Allied Sci 38: 241-247.
- Ellis SF, Pollak AC, Hanson DG, Jiang JJ (1996) Videolaryngoscopic evaluation of laryngeal intubation injury: Incidence and predictive factors. Otolaryngol Head Neck Surg 114: 729-731.
- Hsu CL, Chen KY, Chang CH, Jerng JS, Yu CJ, et al. (2005) Timing of tracheostomy as a determinant of weaning success in critically ill patients: A retrospective study. Crit Care 9: R46-R52.
- Lundy DS, Casiano RR, Shatz D, Reisberg M, Xue JW ( 1998) Laryngeal injuries after short-versus long-term intubation. J Voice 12: 360-365.
- Skoretz SA, Flowers HL, Martino R (2010) The incidence of dysphagia following endotracheal intubation: a systematic review. Chest 137: 665ÃÆÃâÃââÃÆââ¬Å¡Ã¢ââ¬Å¡Ã¬ÃÆââ¬Å¡Ã¢ââ¬Ã
â673.
- Martino R, Foley N, Bhogal S, Diamant N, Speechley M, et al. (2005) Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 36: 2756ÃÆÃâÃââÃÆââ¬Å¡Ã¢ââ¬Å¡Ã¬ÃÆââ¬Å¡Ã¢ââ¬Ã
â2763.
- Stauffer JL, Olson DE, Petty TL (1981) Complications and consequences of endotracheal intubation and tracheotomy: a prospective study of 150 critically ill adult patients. Am J Med 70: 65ÃÆÃâÃââÃÆââ¬Å¡Ã¢ââ¬Å¡Ã¬ÃÆââ¬Å¡Ã¢ââ¬Ã
â76.
- Colice GL, Stukel TA, Dain B (1989) Laryngeal complications of prolonged intubation. Chest 96: 877ÃÆÃâÃââÃÆââ¬Å¡Ã¢ââ¬Å¡Ã¬ÃÆââ¬Å¡Ã¢ââ¬Ã
â884.
- Sue RD, Susanto I (2003) Long-term complications of artificial airways. Clin Chest Med 24: 457ÃÆÃâÃââÃÆââ¬Å¡Ã¢ââ¬Å¡Ã¬ÃÆââ¬Å¡Ã¢ââ¬Ã
â471.
- Santos PM, Afrassiabi A, Weymuller EA (1994) Risk factors associated with prolonged intubation and laryngeal injury. Otolaryngol Head Neck Surg 111: 453-459.
- Cavo JW (1985) True vocal cord paralysis following intubation. Laryngoscope 95: 1352-1359.
- Macht M, King CJ, Wimbish T, Clark BJ, Benson AB, et al. (2013 ) Post-extubation dysphagia is associated with longer hospitalization in survivors of critical illness with neurologic impairment. Crit Care 17: R119
- Hafner G, Neuhuber A, Hirtenfelder S, Schmedler B, Eckel HE (2008) Fiberoptic endoscopic evaluation of swallowing in intensive care unit patients. Eur Arch Otorhinolaryngol 265: 441ÃÆÃâÃââÃÆââ¬Å¡Ã¢ââ¬Å¡Ã¬ÃÆââ¬Å¡Ã¢ââ¬Ã
â446.