Keywords
Antiretroviral therapy; Single tablet regimen; Multiple tablet regimen
Introduction
Combination antiretroviral therapy (ART) has led to significant improvements in HIV-related outcomes and mortality [1]. As a result of increased life expectancy, HIV-infected patients are becoming afflicted with comorbidities that commonly affect the aging population without HIV, including hypertension, diabetes and dyslipidemia [2,3]. These age-related comorbidities are especially problematic for HIV patients, as they are at an elevated risk of adverse cardiometabolic outcomes [4]. The management of HIV infection and comorbidities often requires treatment with multiple agents, resulting in polypharmacy [5]. Polypharmacy, defined as treatment with five or more medications, is associated with reduced adherence and increased mortality among HIVinfected patients [6,7].
In recent years, a number of co-formulated combination ART products have emerged to simplify regimen complexity [8]. Coformulation of ART into single tablet regimen (STR) combination products has reduced the pill burden associated with ART to improve the ease of use for patients and promote medication adherence [3]. Previous studies evaluating STRs have focused on medication adherence and all-cause hospitalizations [9,10]. One study compared adherence to blood pressure and mental health medications to assess relative adherence to ART regimens [11]. However, no previous studies have evaluated non-HIV related outcomes associated with use of STRs.
Currently, there is limited understanding of the relationship between STR use and non-HIV related health outcomes among patients with HIV infection. Specifically, it is unclear if reducing the medication regimen complexity, through use of STRs, may assist HIV-infected patients with non-HIV comorbidity control. When evaluating comorbidity control, there are two populations of interest: those with unmanaged comorbidities requiring achievement of comorbidity control and those whose comorbidities are managed and require maintenance. Within HIV medicine, it is unclear if use of STR affects either achievement or maintenance of comorbidity control. Understanding these relationships is important because it can help clinicians select the most beneficial therapy for patients with a high number of comorbidities and may improve overall health outcomes. Moreover, understanding the relationship between STR use and comorbidity control may have important implications for resource allocation associated with medication therapy management services after STR initiation such as incorporation medication therapy management services by trained professionals.
The purpose of the present study is to compare the frequency of achieving or maintaining cardiometabolic comorbidity control between STR and multiple tablet (MTR) recipients. We hypothesize that HIV-infected patients receiving STRs have a higher probability of achieving or maintaining cardiometabolic comorbidity control than recipients of MTRs.
Materials and Methods
This study was approved by the Institutional Review Board (IRB) of the Stratton Veterans’ Affairs Medical Center with a waiver of informed consent.
Study design and population
A retrospective cohort study was performed among adult HIVinfected Veterans’ Affairs patients who received antiretroviral therapy and medical care between January 2000 and December 2013 within the Upstate New York Veterans’ Affairs Healthcare Network (Veterans Integrated Service Network [VISN] 2). The study employed repeated subject sampling where time/events were apportioned to each ART regimen used by individual patients [12,13]. Patients’ medical records were screened to determine eligibility. Inclusion criteria were: 1) age ≥ 18 years, 2) documented HIV infection, independent of virologic control, 3) received antiretroviral therapy for ≥ 3 months with at least 3 active agents, and 4) had baseline and on-treatment measurements of blood pressure, glucose, lipid laboratory values, or any combination thereof. Patients on incomplete ART regimens, such as fixed-dose monotherapy with zidovudine/abacavir/lamivudine or <3 ART agents, were excluded.
Data collection
Data collected for each subject included demographics, comorbidities, medication history, and select laboratory values. Demographic covariates included age at time of initiation of ART regimen, sex, race, height and weight. Laboratory values and vital health measures were collected serially when available, starting with one measurement before initiation of ART, continuing throughout entire ART regimen and ending at the most recent point of follow up. In patients who may have switched or discontinued their regimen (toxicity, virologic failure, etc.), data collection ended with one value after the termination of ART regimen, if available. Laboratory values extracted from the patients’ medical records included basic metabolic and lipid panels. The only vital health status measure collected for this study, was blood pressure (BP), assessed by two consecutive blood pressure measurements. For patients hospitalized at any point during the study period, only outpatient laboratory/BP values were utilized.
Medication histories were documented for all ART and non-ART medications at the time of starting STR or MTR regimen. The specific data elements collected for medication histories included drug name, dose, dosing frequency and duration of therapy. The total number of concomitant medications during each sampled regimen was determined. For the purposes of these analyses, the terms product and tablet are synonymous. Similarly, the terms agent and medication are synonymous and refer to a specific medication component (e.g. zidovudine/abacavir/lamivudine is a fixed dose tablet/product that contains three antiretroviral agents/medications). Regimen refers to the complete set of antiretroviral agents/medications used simultaneously to treat an individual’s HIV infection, which may or may not be coformulated in the same product/tablet.
Use of single tablet or multiple tablet art regimens
The exposure of interest in this study was the use of STR or MTR ART regimens. To define the use of STR or MTR, pill burden for each regimen was determined. Patients with an ART pill burden equal to one were considered STR recipients, while patients with an ART pill burden >1 were considered MTR recipients.
Outcomes
The primary outcomes of this study were control of BP, glucose, and/or lipids. Blood pressure control was defined according Joint National Commission- 8 (JNC-8) BP goals (two consecutive non-hospitalized blood pressure readings <150/90 mm Hg for individuals age ≥ 60 years or <140/90 mm Hg for all others) [14]. Glycemic control was defined according to the American Diabetes Association Standards of Medical Care in Diabetes (two consecutive non-hospitalized fasting plasma glucose values <126 mg/dL) [15]. The Adult Treatment Panel III Guidelines from the National Cholesterol Education Program was used to define lipid control [16].
Because some patients had already achieved cardiometabolic comorbidity control or various components of control upon initiation of STR or MTR regimen, patients were partitioned into one of six subpopulations corresponding to each study outcome: those with control of 1) blood pressure, 2) glycemic, or 3) lipid values and those lacking control of 4) blood pressure, 5) glycemic, or 6) lipid values at the time of initiating ART regimen. Patients categorized into comorbidity control subpopulations at ART regimen initiation were required to have had prior diagnosis with the cardiometabolic comorbidity for the respective outcome based on their problem list. For the first three subpopulations, the outcome of interest was maintenance of comorbidity control defined as never having two consecutive measurements (one for lipids) outside of the range for comorbidity control at any point during the ART regimen. For the latter three subpopulations, the outcome of interest was achievement of comorbidity control defined as having two consecutive measurements (one measurement for lipids) within the range for comorbidity control at any point during the ART regimen.
Statistical analyses
Univariate analyses were performed using descriptive statistics. Among each of the 6 subpopulations, bivariate analyses were performed comparing STR/MTR status with clinical/demographic characteristics. A second set of bivariate analyses were performed comparing clinical/demographic characteristics with each outcome. For categorical variables, chi-square or Fisher’s exact tests were performed. For continuous variables, Student’s t-test or Mann Whitney U tests were used, as appropriate. Stratified analyses were performed to assess effect modification in the bivariate analyses and compared using the Breslow-Day test. Given the low sensitivity of the test, a p-value threshold of 0.2 was used to denote significance for this test only. A p-value <0.05 was considered statistically significant for all other tests. Classification and regression tree (CART) analyses were performed to identify breakpoints in continuous variables associated with each of the study outcomes.
To determine if use of STR/MTR was independently associated with each of the study outcomes, multivariate Cox proportional hazards (PH) regression analyses were performed. Variables that were associated with study outcomes (p<0.25) and were present in >5% of the study population were eligible for entry into the Cox PH regression models as potential confounders. If the resulting hazard ratio for STR was altered by >10%, the variable was retained in the final model as a confounder. The process was repeated until all potential confounders were assessed. Effect modification was assessed through the use of interaction terms in the Cox PH regression models. All data were analyzed in SAS v9.3 (Cary, NC, USA) and SPSS v21 (Chicago, NY, USA).
Results
Patient disposition and characteristics
There were a total of 1,202 subjects, derived from 562 unique patients, who received either a STR (13.7%) or MTR regimen during the study period. The median (interquartile range, IQR), number of regimens utilized was 2 (1-3). The mean ± standard deviation (SD) age of subjects was 50.6 ± 8.9 years. The majority of subjects were male (97.2%) with a similar distribution of Caucasian (45.8%) and Black (49.8%) races. The median (IQR) number of non-HIV medications was 8 (4-12). The median (IQR) number of comorbidities was 14 (8-21). The distribution of regimen type among the MTR recipients was PI (42.1%); mixedclass (36.8%), NNRTI (27.7%) and the remainder were INSTI (3.4%). The majority of STR regimens were NNRTI-based (98.2%). Patient demographics and clinical characteristics for STR/MTR recipients are presented in Table 1. The only variables that differed between STR and MTR recipients were age and risk behavior.
| Covariate |
Multiple tablet regimen recipients
(n=1037) |
Single tablet regimen recipients
(n=165) |
P-value |
| Age, mean (standard deviation, SD) |
50.3 ± 8.8 |
53.0 ± 9.0 |
<0.001 |
Race
- Caucasian
- Black
- Hispanic
- Asian/Pacific Islander
- Other
|
473 (45.6)
519 (50.0)
33 (3.2)
3 (0.3)
9 (0.9) |
77 (46.7)
80 (48.5)
6 (3.6)
1 (0.6)
1 (0.6) |
0.95 |
| Sex, male (%) |
1010 (97.4) |
158 (95.8) |
0.24 |
Risk behavior
- MSM
- MSM/IVDU
- IVDU
- Heterosexual sex
- Female-female
- Unknown
|
252 (24.3)
57 (5.5)
299 (28.8)
328 (21.6)
2 (0.2)
99 (9.5) |
37 (22.4)
8 (4.8)
31 (18.8)
74 (44.8)
0 (0)
15 (9.1) |
0.02 |
| Number of comorbidities, median (IQR) |
14 (8-21) |
15 (9-21) |
0.42 |
| Blood pressure medications |
283 (44.6) |
55 (45.1) |
0.92 |
| Oral anti-diabetic medications |
39 (5.9) |
7 (5.6) |
0.91 |
| Insulin |
15 (2.3) |
4 (3.2) |
0.53 |
| HMG CoA Reductase inhibitors (statins) |
75 (23.5) |
19 (27.5) |
0.54 |
Table 1: Bivariate relationship between clinical/demographic characteristics and single/multiple tablet regimen.
Maintenance of Comorbidity Control
Maintenance of BP control among subjects with normal BP at regimen initiation
There were 370 subjects (60 STR; 310 MTR) with prior diagnosis of hypertension and controlled BP at commencement of ART regimen with on-treatment values available for analysis. There were 272 (73.5%) subjects receiving concomitant anti-hypertensive therapy. Maintenance of BP control was not significantly lower for recipients of STRs than MTRs (50.0% versus 65.5%, p=0.44) (Figure 1). There did not appear to be effect modification upon stratification by tobacco use status (Breslow-Day p=0.41). Among non-smokers, a similar proportion of STR recipients maintained BP control relative to MTR recipients (68.8% versus 64.0%, p=0.79). Among smokers, similar proportions were observed between STR and MTR subjects maintaining BP control (43.2% and 51.4%, p=0.32). There did not appear to be effect modification upon stratification by use of anti-hypertensive medications (Breslow- Day p=0.27). Among subjects not using anti-hypertensive medications, the proportion of STR subjects that maintained BP control relative to MTR subjects did not significantly differ (61.5% versus 77.6%, p=0.30). Among recipients of antihypertensive medications, the proportion of subjects maintaining BP control did not differ between STR and MTR users (46.8% versus 47.1%, p=0.97). In multivariate analyses, STR use (hazard ratio, HR: 1.19, 95% confidence interval, CI: 0.77-1.86, p=0.44) was not significantly associated with loss of maintenance of BP control after adjustment of concomitant use of thiazide diuretics, vitamins/minerals, abacavir, tobacco, cocaine, alcohol and age.
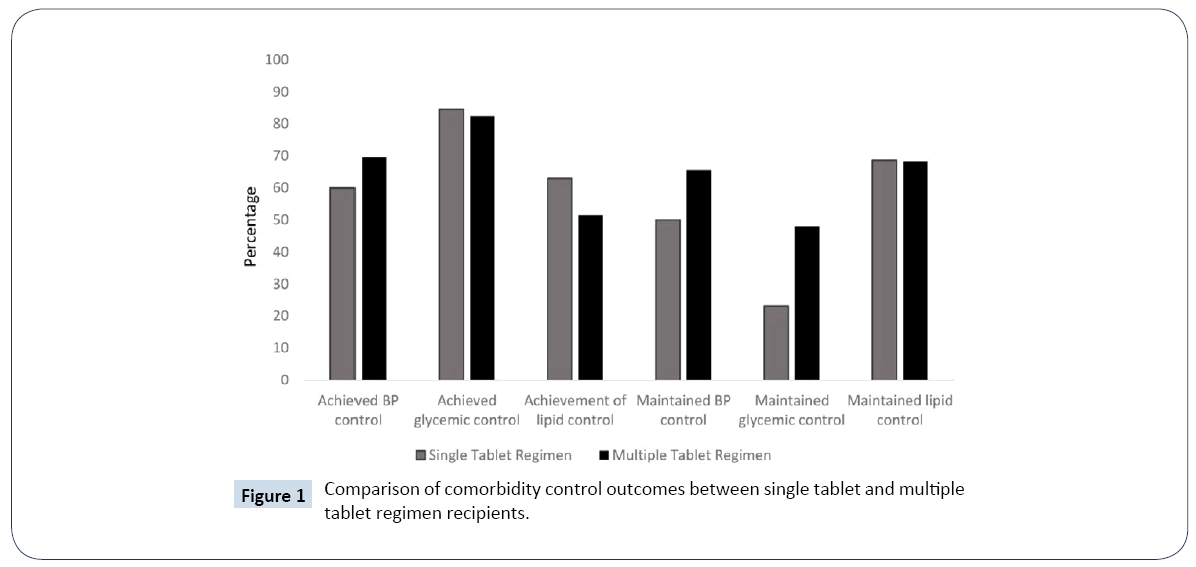
Figure 1: Comparison of comorbidity control outcomes between single tablet and multiple tablet regimen recipients.
Maintenance of glycemic control among subjects with normal blood glucose at regimen initiation
Among the 115 subjects with prior diagnosis of diabetes in their problem list and normal baseline glucose values (80-130 mg/ dL), there were 102 MTR and 13 STR recipients. Use of insulin products and oral anti-diabetic medications were observed in 19 (16.5%) and 39 (33.9%) of subjects in this subgroup, respectively. Maintenance of glycemic control (<130 mg /dL) did not significantly differ between STR and MTR recipients (23.1% versus 48.0%, p=0.14) (Figure 1). Furthermore, the relationship between STR use and maintenance of glycemic control was not modified by use of insulin or oral anti-diabetic medications. In the multivariate analysis, STR was not independently associated with loss of glycemic control (HR: 1.83, 95% CI: 0.81-4.12, p=0.15) after adjustment for concomitant use of insulin products, oral anti-diabetic medications, protease inhibitors and age.
Maintenance of lipid control among subjects with normal lipids at regimen initiation
Among the 177 subjects with prior dyslipidemia diagnosis and normal baseline lipids, there were 35 STR recipients and 142 MTR recipients. Maintenance of lipid control did not differ between STR and MTR recipients (68.6% versus 68.3%, p=0.98) (Figure 1). No difference in maintenance of lipid control was observed between STR and MTR recipients when stratified by those using of any lipid lowering therapy (55.0% versus 60.5, p=0.65) and not using lipid lowering therapy (86.7% versus 80.4%, p=0.72) (Breslow-Day p=0.48). In the multivariate analyses, use of STR (HR:0.84, 95% CI:0.40-1.77, p=0.65) was not independently associated with loss of lipid control after adjustment for age, sex, use of lipid lowering therapy, protease inhibitors, oral anti-diabetic medications, non-steroidal anti-inflammatory drug (NSAID)/cyclooxygenase-2 (COX-2) inhibitor use, and tobacco.
Achievement of Comorbidity Control
Achievement of controlled BP among subjects with abnormal bp at regimen initiation
There were 178 subjects (30 STR; 148 MTR) with a prior diagnosis of hypertension and uncontrolled BP at commencement of ART regimen. The median [interquartile range (IQR)] systolic (SBP) and diastolic (DBP) values at baseline were 149 (141-156) and 92 (83-97) mm Hg, respectively. Median (IQR) SBP was lower in STR recipients compared to MTR recipients (144 (136-151) versus 150 (142-157), p=0.05), but median (IQR) DBP did not differ between STR and MTR recipients (93 (82-99) versus 91 (84-97), p=0.99). Concomitant anti-hypertensive use was observed in 146 subjects (28 STR, 118 MTR). Achievement of BP control did not significantly differ between STR and MTR recipients (60.0% versus 69.6%, p=0.30) (Figure 1). In multivariate analyses, STR use (HR: 1.22, 95% CI: 0.71–2.09, p=0.48) was not significantly associated with achievement of BP control after adjustment of concomitant use of anti-hypertensive agents, tobacco, heroin and age.
Achievement of glycemic control among subjects with abnormal blood glucose at regimen initiation
For 70 subjects with a prior diagnosis of diabetes in their problem list, abnormal baseline glucose values and on-treatment glucose values for evaluation, there were 13 STR and 57 MTR recipients. A higher proportion of STR recipients were concomitantly using insulin products than MTR subjects (69.2% versus 33.3%, p=0.03). In contrast, the use of oral anti-diabetic agents was not significantly different among STR subjects than MTR recipients (69.2% versus 54.4%, p=0.37). Achievement of glycemic control was similar between STR and MTR recipients (84.6% versus 82.5%, p=1.00) (Figure 1). After stratification by use of insulin products, there did not appear to be effect modification of the relationship between STR/MTR and achievement of glycemic control among recipients (77.8% versus 68.4%, p=0.61) and non-recipients (100% versus 89.5, p=1.00) of insulin products (Breslow-Day p=0.59). There was also no relationship between STR/MTR use and achievement of glycemic control among users (88.9% versus 83.9%, p=1.00) and non-users (75% versus 80.8%, p=1.00) of oral anti-diabetic medications (Breslow-Day p=0.65). In the multivariate analyses, the use of STR (HR: 1.10, 95% CI: 0.52-2.31, p=0.81) was not independently associated with achievement of glycemic control after adjustment for use of insulin products, oral anti-diabetic medications, protease inhibitors, age and tobacco.
Achievement of lipid control among subjects with abnormal lipids at regimen initiation
Among the 128 subjects with a prior diagnosis of dyslipidemia, abnormal baseline lipids and on-treatment lipids available for evaluation, STRs were used by 27 subjects. Achievement of lipid control did not statistically differ between STR and MTR recipients (63.0% versus 51.5%, p=0.29) (Figure 1). Among subjects not using any lipid lowering therapies, there did not appear to be a significant difference between STR/MTR use and achievement of lipid control (68.4% versus 58.2%, p=0.60). There was no significant difference in achievement of lipid control among users of lipid-lowering therapies between STR and MTR recipients (50.0% versus 38.2%, p=0.69), (Breslow-Day=0.97). In multivariate analyses, STR use (HR: 0.91, 95% CI: 0.50-1.67, p=0.76) was not significantly associated with achievement of lipid control after adjustment for adjustment for age, sex, use of lipidlowering therapy, protease inhibitors and tobacco.
Discussion
Overall, the results of this study do not indicate significant differences in either achieving or maintaining control of cardiometabolic comorbidities between recipients of STRs and MTRs after adjustment for confounding variables. This differs from our hypothesis that STR recipients would have better comorbidity control overall and from previous studies that suggest that STR recipients have better medication adherence [9,10]. Instead, STR and MTR recipients generally do not appear to significantly differ in either achieving or maintaining comorbidity control after adjustment for confounding factors. Thus, comorbidity control should not be the sole deciding factor for clinicians to prescribe a STR or MTR. However, previous studies have observed patient preference for STRs, resulting in improved outcomes [3,9,17,18]. Ultimately, consideration of patients’ motivation and virologic control should still be primary factors for clinicians selecting an ART regimen.
Some limitations of the present study should be considered when interpreting these data. First, low numbers of subjects were available for analysis when divided into the six subpopulations, especially STR subpopulations. Thus, the limited observation of statistical differences may have been due, in part, to power. This was particularly true for stratified analyses within these 6 subpopulations. Future studies should corroborate these findings in larger cohorts of patients with sufficient numbers of subjects within strata of important modifying variables. Second, the generalizability of our findings may be limited by our study population. The U.S. Veteran HIV patient population may have comorbidities, risk behaviors, access to care and other characteristics that differ from other HIV-infected patient populations. Conversely, collection of data from Veterans’ Affairs medical records represents a significant strength in data quality. Third, our study did not seek to evaluate heterogeneity within ART regimen types. There is significant variability among MTR regimens by drugs included and frequency of dosing regimen. Differences in adherence have previously been observed between MTR regimens dosed once and twice daily [18]. Our study did not assess the impact of this varying regimen complexity, particularly adherence, within the MTR population, as no significant differences in comorbidity control were observed between STR and MTR populations in bivariate analysis. Many antiretroviral drugs, especially older classes of drugs, have significant metabolic adverse effects as well as potential interactions with agents used in the treatment of cardiometabolic comorbidities [19,20]. Our study was not powered to observe differences in outcomes between drugs classes utilized in MTR patients; thus, unobserved class-specific effects on cardiometabolic outcomes may have influenced these results. Newer drug formulations have generally demonstrated more favorable metabolic profiles [20]. Many of these formulations have only become commercially available recently and were not available for the majority of the study period. Notably, there were no STR formulations available during the study period until efavirenz/emtricitabine/ tenofovir disoproxil fumarate became available in 2006 [21]. This formulation was utilized by most STR patients in our study population. However, regimens containing efavirenz have been associated with select adverse effects and reduced therapy longevity compared to STR formulations that have subsequently become available [20,22]. Furthermore, newer formulations have shown improved adherence and are increasingly being utilized [23]. Fourth, achievement/maintenance of lipid/glycemic/blood pressure control did not necessarily capture or reflect sustained control. Patients could have achieved/maintained control of any of the outcomes and then reverted to an uncontrolled state at a later point in therapy. Finally, by utilizing medical records in a retrospective study design, we were unable to assess lifestyle factors that may have affected patient outcomes.
Conclusion
In summary, we did not observe a meaningful difference between STR/MTRs and patients’ ability to achieve/maintain control of HIV comorbidities after adjustment for confounding factors. Future studies should continue to seek further understanding of the impact of ART regimen type on both HIV and non-HIV health outcomes, with greater inclusion of modern STR products that may have more favorable adverse effect profiles.
Acknowledgement and Disclosures
1. This study was approved the Institutional Review Board (IRB) of the Stratton Veterans’ Affairs Medical Center with a waiver of informed consent.
2. This material is based upon work partially supported by the Office of Research and Development, Department of Veterans Affairs. The work presented in this manuscript was also partially funded by an investigator-initiated research grant from Gilead Sciences. The contents of this report do not represent the views of the Department of Veterans Affairs or the United States Government.
3. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published.
4. Dr. Patel has received investigator-initiated research grant support from Gilead Sciences and Merck & Co. None of the other authors have conflicts of interest to declare, including, but not limited to, consulting fees, paid expert testimony, employment, grants, honoraria, patents, royalties, stocks or other financial or material gain that may involve the subject matter of the manuscript.
References
- Vittinghoff E, Scheer S, O'Malley P, Colfax G, Holmberg SD, et al. (1999) Combination antiretroviral therapy and recent declines in AIDS incidence and mortality. J Infect Dis 179: 717-720.
- Awofeso N, Rammohan A (2010) AIDS and elderly people. Elderly Africans have AIDS too. BMJ 341: c4169.
- Sterrantino G, Santoro L, Bartolozzi D, Trotta M, Zaccarelli M (2012) Self-reported adherence supports patient preference for the single tablet regimen (STR) in the current cART era. Patient Prefer Adherence6: 427-433.
- Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, et al. (2011)Morbidity and aging in HIV-infected persons: The Swiss HIV cohort study. Clin Infect Dis 53: 1130-1139.
- Koram N, Vannappagari V, Sampson TJ, Panozzo CA (2014) Non-ARV Prescriptions and Medication Burden among Commercially Insured U.S. HIV Patients. Journal of Pharmaceutics and Drug Development 2.
- Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, et al. (2013) The next therapeutic challenge in HIV: polypharmacy. Drugs Aging 30: 613-628.
- Kodama F, Skalweit M, Burant C, Hirsch A (2013) Differences in calculated adherence rates of ART and non-ART medications among HIV positive veterans. IDweek San Francisco, CA.
- Sebaaly JC, Kelley D (2017) Single-tablet regimens for the treatment of HIV-1 Infection. Ann Pharmacother 51: 332-344.
- Sutton SS, Hardin JW, Bramley TJ, D'Souza AO, Bennett CL (2016) Single- versus multiple-tablet HIV regimens: Adherence and hospitalization risks. Am J Manag Care 22: 242-248.
- Cohen CJ, Meyers JL, Davis KL (2013) Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US medicaid population with HIV. BMJ Open 3.
- Langness J, Cook PF, Gill J, Boggs R, Netsanet N (2014) Comparison of adherence rates for antiretroviral, blood pressure, or mental health medications for HIV-positive patients at an academic medical center outpatient pharmacy. J Manag Care Spec Pharm 20: 809-814.
- Mitchell ML, Jolley JM (2009) Research design explained (7th Ed.). Belmont, CA: Wadsworth Cengage Learning.
- Howell DC (2009) Statistical methods for psychology (7th Ed.). Belmont CA: Wadsworth Cengage Learning.
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, et al. (2014) Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507-520.
- Chamberlain JJ, Rhinehart AS, Shaefer CF Jr., Neuman A (2016) Diagnosis and management of diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med 164: 542-552.
- National Cholesterol Education Program Expert Panel on Detection E (2002) Treatment of High Blood Cholesterol in A: Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143-3421.
- Airoldi M, Zaccarelli M, Bisi L, Bini T, Antinori A, et al. (2010) One-pill once-a-day HAART: A simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Prefer Adherence 4: 115-125.
- Parienti JJ, Bangsberg DR, Verdon R, Gardner EM (2009) Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis 48: 484-488.
- Fichtenbaum CJ, Gerber JG (2002) Interactions between antiretroviral drugs and drugs used for the therapy of the metabolic complications encountered during HIV infection. Clin Pharmacokinet 41: 1195-1211.
- Srinivasa S, Grinspoon SK (2014) Metabolic and body composition effects of newer antiretrovirals in HIV-infected patients. Eur J Endocrinol 170: 85-202.
- Atripla [package insert] (2016). Foster City, CA: Bristol-Myers Squibb & Gilead Sceinces LLC.
- Sweet D, Song J, Zhong Y, Signorovitch J (2014) Real-world medication persistence with single versus multiple tablet regimens for HIV-1 treatment. J Int AIDS Soc 17: 19537.
- Viswanathan S, Detels R, Mehta SH, Macatangay BJ, Kirk GD, et al. (2015) Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART). AIDS Behav 19: 601-611.