Research Article - (2024) Volume 32, Issue 1
Received: 09-Jan-2024 Editor assigned: 11-Jan-2024 Reviewed: 25-Jan-2024 Revised: 30-Jan-2024 Published: 06-Feb-2024, DOI: 10.36648/1479-1064.32.1.03
Background: Acute Lymphoblastic Leukemia (ALL) is the commonest childhood cancer globally. We described the clinical features at diagnosis and established the overall survival of children diagnosed with ALL at our Pediatric Cancer Unit.
Methods: In August 2020, we retrospectively studied children <16 years diagnosed with ALL, over a 4-year period (June 2016 to May 2020) at Mbarara Regional Referral Hospital (MRRH) in South West Uganda. Frequencies and proportions of baseline clinical features and treatment outcomes were described. Kaplan-Meier analysis and Cox proportional hazard regression model were performed to estimate overall survival and identify its predictors respectively. Ethical approval was obtained from Research Ethics Committee of Mbarara University of Science and Technology and waiver given for consent.
Results: Within the 4-year period, 301 children were diagnosed with cancer; 51 (16.9%) with ALL. 44 (86.3%) presented with fever, 28 (54.9%) cough, 21 (41.2%) bleeding tendencies, 20 (39.4%) limb pains and 8 (15.7%) abdominal distension. 44 (86.3%) had pallor, 39 (76.5%) lymphadenopathy, 37 (72.5%) hepatosplenomegaly, 18 (35.3%) pyrexia, 12 (23.5%) bone tenderness and 11 (21.6%) petechia. Thirty (58.8%) children presented with leukocytosis (WBC>12 × 109/L), all the children had anemia (Hb) <11.0g/dl) and 48 (94.1%) had thrombocytopenia (<150.0 × 109/L). 33 (64.7%) children completed induction chemotherapy; 27 (81.8%) with remission. Overall one year survival was 42.5%. Remission failure was associated with poor survival.
Conclusion and recommendation: Children with ALL present with non-specific clinical features that mimic common childhood infections and its outcomes are low at our unit. ALL should form part of the differential diagnosis in children with fever, pallor, bleeding, or leukocytosis, anemia and thrombocytopenia.
Acute lymphoblastic leukemia; Central nervous system; Cerebral spinal fluid; Hemoglobin; White blood cell count
Acute leukemia is the commonest cancer among children representing about 25% of all childhood cancers worldwide. Acute Lymphocytic Leukemia (ALL) is the most common leukemia, accounting for approximately 75% of all childhood acute leukemia [1].
Children with acute lymphoblastic leukemia present with a variety of clinical features that are usually rapid in onset and progression [2]. Studies done in Rwanda, Tanzania, Uganda, Malawi, Europe and North America reported that children with ALL commonly present with body weakness, fever, epistaxis, bleeding gums, bone pain, vomiting and a history of multiple blood transfusions [2-7]. Without sufficient laboratory support, acute leukemia can easily be confused with common childhood infectious illnesses, such as malaria and tuberculosis, especially in the developing world, where the knowledge regarding cancer is low among health care workers and infectious diseases that may mimic ALL are common [2]. With the limited knowledge, there is delayed diagnosis and high rates of cancer underdiagnoses among children in the developing world [1,8,9].
There is limited data on the survival of children with ALL in Sub-Saharan Africa. Some studies done reported 1-year overall survival rates of 66%, 46.3%, and 84.6% and 75% in Tanzania, Zambia, Kenya and Uganda, respectively [10-13]. This is in contrast to high 5-year over-all survival rates of up to 85% in the developed world owing to early diagnosis, improved supportive care and a risk adapted therapy which are not available in the developing world [1,14-17].
Treatment for acute lymphoblastic leukemia has been ongoing at the Pediatric Cancer Unit (PCU) of Mbarara Regional Referral Hospital (MRRH) since 2016. The aim of this study was describing clinical profiles at admission and overall survival of children with ALL. This will help to create awareness of the clinical presentations of ALL to the caretakers. This will in turn lead to early health seeking behaviors, early diagnosis and treatment initiation. Primary clinicians will also be educated about the common clinical features and laboratory findings that children with ALL present with to enable them to quickly investigate and initiate early treatment for better outcomes.
Study Site
The study was done at the PCU of MRRH, situated in Mbarara Municipality about 300 km southwest of the Ugandan capital, Kampala. MRRH is the teaching hospital of Mbarara University of Science and Technology (MUST). The PCU is a 16-bed capacity ward and outpatient clinic that treats children with cancer below 16 years of age and is headed by a pediatric oncologist.
Diagnosis and Treatment
Children suspected to have cancer undergo clinical assessment through history taking, physical examination and laboratory investigations. Bone marrow aspirate, biopsy and CSF samples are then taken off by the clinic staff and transported and processed by a private laboratory.
Once the diagnosis is confirmed by either morphology or flow cytometry, the children are enrolled into care and their demographic data, clinical features, risk group, and results of other investigations entered into paper-based and electronic records. The Berlin-Frankfurt-Munster 96 (BFM 96) protocol was used to treat all the children with ALL [18].
Study Design
This was a retrospective review of all the medical records of children diagnosed with ALL from 1st June 2016 to 31st May 2020. Data was extracted to include social-demographics, clinical and laboratory features at admission, mode of diagnosis, presence of CNS involvement, National Cancer Institute (NCI) risk group, nutrition status, end of induction remission status and vital status (alive, dead or loss to follow up). Overall survival (OS) was calculated from the time of confirmed diagnosis up to death from any cause [19].
The data was entered into EPI-INFO® version 7.2 and appropriate data cleaning and verification processes were implemented. The data was then exported to STATA® version 14.0 for analysis. Continuous data was summarized into means, standard deviations, median and interquartile ranges. Proportions for categorical or binary data were done and results presented as percentages. The overall survival was calculated using Kaplan-Meier curve and expressed as a percentage with its corresponding 95% confidence interval. Both univariate and multivariable analysis cox proportional hazard model regression were done to establish variables associated with overall survival. The unadjusted and adjusted hazard ratios with their corresponding 95% confidence intervals were reported for each covariate and a significance level of 5% was considered. Ethical approval was obtained from the Research Ethics Committee (REC) of Mbarara University of Science and Technology and a waiver for consent given.
301 children were diagnosed with cancer over the study period. In Figure 1, 81 children (26.9%) was diagnosed with leukemia. Of these, 51 (66.2%), 26 (33.8%) and 4 (4.9%) had acute lymphoblastic leukemia, acute myeloid leukemia and chronic myelogenous leukemia respectively. All the 51 children with acute lymphoblastic leukemia met the inclusion criteria and were enrolled into the study.
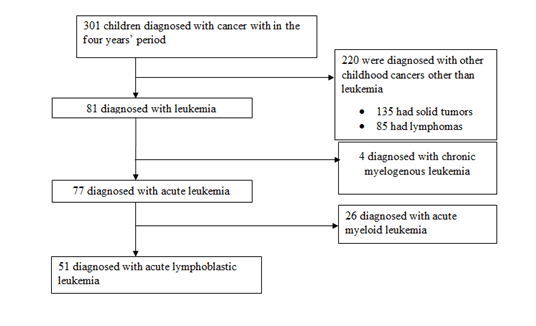
Figure 1: Flow chart summarizing patient numbers in the study
Demographic and Clinical Characteristics of Children with ALL at Admission
As shown in Table 1, 29 (56.9%) children were male with a median (range) age of 7.08 (1-15) years. Their median (range) duration of onset of symptoms before diagnosis was 8 (1-24) weeks and the median (range) time between admission and diagnosis was 2 (0-12) days.
Table 1: Demographic and clinical features at admission of children diagnosed with ALL at MRRH cancer unit
| Clinical features | Frequency (n) | Percentage (%) |
|---|---|---|
| Sex, Male | 29 | 56.9 |
| Age (years), 0-9 | 37 | 72.5 |
| Age (years), 10-15 | 14 | 27.5 |
| Fever | 44 | 86.3 |
| Cough | 28 | 54.9 |
| Bleeding tendencies | 21 | 41.2 |
| Limb pain | 20 | 39.2 |
| Abdominal distension | 8 | 15.7 |
| Abdominal pain | 4 | 7.8 |
| Pallor | 44 | 86.3 |
| Lymphadenopathy | 39 | 76.5 |
| Hepatosplenomegaly | 37 | 72.5 |
| Pyrexia | 18 | 35.3 |
| Bone tenderness | 12 | 23.5 |
| Petechia | 11 | 21.6 |
| Mediastinal mass | 10 | 19.6 |
| Severe acute malnutrition | 2 | 3.9 |
The children presented with variable clinical features at admission as summarized in Table 1, the most prevalent symptoms being 44 (86.3%) fever and 28 (54.9%) cough. Pallor 44 (86.3%) and lymphadenopathy 39 (76.5%) being the most common signs.
Laboratory Features of Children with ALL at Admission
The laboratory features of the children at admission are shown in Table 2. The median (range) white blood cell count at admission was 31.78 (0.88-552.95) × 106/L. All children with ALL had anemia (Hb<11.0g/dl) at admission, with a median Hb of 5.6g/dl. The median (range) platelet count of 35 (1-544) × 106/L. Data on CNS disease status was available for only 20 (39.2%) children. Of these, 2 (10.0%) were positive.
Samples used for diagnosis were peripheral blood among 8 (15.7%) children and bone marrow biopsy among 43 (83.3%). 45 (88.2%) children were diagnosed by morphology alone, 5 (9.8%) by both flow cytometry and morphology and 1 (2.0%) by flow cytometry alone; 2 (3.9%) had T-cell ALL and 4 (7.8%) B-cell. Using the NCI risk stratification criteria for ALL, 25 (49.0%) children were standard risk and 26 (51%) were high.
Table 2: Laboratory features, diagnosis, ALL subtypes and NCI risk stratification at admission among children with ALL at MRRH cancer unit
| Frequency(n) | Percentage (%) | ||
|---|---|---|---|
| Laboratory findings (n=51) | |||
| WBC Ã? 109/L | <50 | 36 | 70.6 |
| â?¥ 50 | 15 | 29.4 | |
| Hemoglobin g/dl | <7 | 32 | 62.7 |
| 7-11 | 19 | 37.3 | |
| Platelet Ã? 109/L | <50 | 32 | 62.7 |
| 50-150 | 16 | 31.4 | |
| 150-450 | 3 | 5.9 | |
| CNS disease (n=20) | Positive | 2 | 10 |
| Negative | 18 | 90 | |
| Diagnostic sample used (n=51) | |||
| Peripheral blood | 8 | 15.7 | |
| Bone marrow biopsy | 43 | 84.3 | |
| Mode of diagnosis of ALL(n=51) | |||
| Morphology only | 45 | 88.2 | |
| Flow cytometry and Morphology | 5 | 9.8 | |
| Flow cytometry only | 1 | 2 | |
| Subtypes of ALL(n=6) | |||
| T-cell ALL | 2 | 33.3 | |
| B-cell ALL | 4 | 66.7 | |
| Risk stratification of ALL (n=51) | |||
| Standard risk | 25 | 49 | |
| High risk | 26 | 51 | |
Induction Outcomes
Of the 51 children initiated on treatment, 12(23.5%) died, 6 (11.8%) abandoned treatment before day 29 of induction chemotherapy and 33(63.7%) completed induction chemotherapy. As shown in Figure 2, of the children who completed induction chemotherapy, 27 (81.82%) achieved full remission.
Overall Survival
As shown in Figure 3, the one, two, three and four-year overall survival of children diagnosed with acute lymphoblastic leukemia were 42.5%, 23.5%, 23.5% and 23.5% respectively.
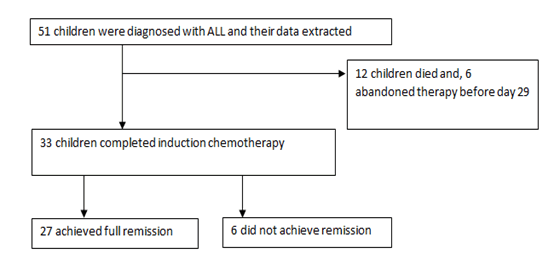
Figure 2: Induction outcomes among children with acute lymphoblastic leukemia at MRRH cancer unit
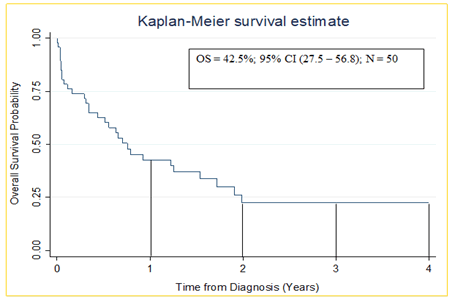
Figure 3: Kaplan-Meier Estimate (1 year overall survival) of children diagnosed with acute lymphoblastic leukaemia at MRRH cancer unit
Predictors of One-Year Overall Survival
At univariate analysis, the hazard of death was inversely associated with hemoglobin levels below 7.0 g/dl (HR: 0.42, 95% CI: 0.19-0.89, p=0.025). Also, failure to achieve morphological remission was associated with an increased hazard of death (HR: 0.41, 95%CI: 0.19-0.84, p=0.015). At multivariable analysis, failure to achieve remission was the only factor associated with reduced one-year overall survival with hazard of death (aHR: 0.43, 95%CI: 0.19-0.97, p=0.042), as shown in Table 3.
Our study found a one-year overall survival of 42.5%. This is inferior to outcomes reported at Uganda Cancer Institute (UCI), Tanzania, and Nairobi with overall survival at 75%, 66% and 84.6% respectively [10-13]. A potential explanation for the inferior outcomes observed from our Center is the lack of flow cytometry and cytogenetics to inform risk adjusted therapy (intensified for T cell, MRD high risk and adverse mutations). It may also be due to better supportive care resources in those centers. 1 year overall survival was low in our study compared to High Income Countries (HIC) where it is currently over 85% [1]. The reason for this difference is, partly attributed to early diagnosis, improved supportive care, more precise risk stratification, and risk adapted chemotherapy informed by disease biology which are not readily available in the LMIC [17,20]. It is also reported that under diagnosis/late diagnosis, treatment abandonment, coexisting comorbidities such as malnutrition and infections, suboptimal supportive and palliative care, and inefficient health care delivery systems represent major limitations to pediatric cancer care in LMIC [13,21]. Such factors may have contributed to the low overall survival among children with ALL in our setting.
Table 3: Multivariable Cox proportional hazards predictors of one-year overall survival among children diagnosed with acute lymphoblastic leukemia at MRRH cancer unit
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p-value | HR | (95% CI) | p-value | |
| Sex | ||||||
| Female | Ref | (0.19-1.26) | 0.137 | Ref | (0.03-1.04) | 0.056 |
| Male | 0.48 | 0.18 | ||||
| Age of child (years) | ||||||
| 0-9 | Ref | (0.28-1.14) | 0.112 | Ref | (0.22-1.21) | 0.127 |
| 10-16 | 0.56 | 0.52 | ||||
| Risk Group | ||||||
| High risk | Ref | (0.59-2.45) | 0.6 | Ref | (0.04-1.81) | 0.179 |
| Standard Risk | 1.21 | 0.27 | ||||
| White blood cell count × 109/L | ||||||
| ≥50 | Ref | Ref | (0.49-19.33) | 0.229 | ||
| <50 | 0.86 | (0.40-1.83) | 0.692 | 3.09 | ||
| Hemoglobin count, g/dl | ||||||
| ≥7.0 | Ref | Ref | (0.23-1.30) | 0.176 | ||
| <7.0 | 0.42 | (0.19-0.89) | 0.025* | 0.55 | ||
| Platelet count × 109/L | ||||||
| ≥50 | Ref | (0.45-1.95) | 0.869 | - | - | - |
| <50 | 0.94 | - | - | - | ||
| Morphological Remission | ||||||
| Yes | Ref | (0.19-0.84) | 0.015* | Ref | (0.19-0.97) | 0.042* |
| No | 0.41 | 0.43 | ||||
| *significant at p=<.05 | ||||||
From our study, we found that children with ALL had a varied clinical presentation. The children all presented with typical clinical features of ALL with fever 44 (86.3%), and pallor 44 (86.3%) being the most common as it is reported in other studies [4-6,22] Health workers may have been inclined to explore and treat other more common causes of fever and pallor, hence leading to diagnostic delay.
Our study found out that 15 (29.4%) children presented with WBC ≥ 50 × 109/L. The median (range) white blood cell count at admission was 31.78 (0.88-552.95) × 109/L. This is similar to the findings from a study in Pakistan where 182 (28.8%) had a WBC ≥ 50 × 109/L [23]. Our study had more children present with a WBC ≥ 50 × 109/L than those in Brazil where only 21% children had WBC ≥ 50 × 109/L. This difference may be attributed to early diagnosis among children with ALL in Brazil as compared to our setting. However the findings are not different from what has been reported internationally where about 20% of children with Acute Lymphoblastic Leukemia present with a high WBC above 50,000/mm3 [5,24]. In our study all children with ALL had anemia (Hb<11.0g/dl) at admission. The subjects in our study had a lower median Hb than that reported from other studies. 5.6 (0.9-10.7) g/dl versus 6.5 g/dl, 7.7 g/dl and 8.24 from UCI, Pakistan and Brazil respectively [5,6,23]. This may probably be explained by the delays in early seeking of treatment. In our study, 48 (94.1%) of the children had thrombocytopenia (<150.0 × 109/L) with the median (range) platelet count of 35(1- 544) × 109/L. This is not different from other reports in Brazil and Uganda [5,7]. Two children (10.0%) had central nervous system involvement. This percentage was higher than in a study done in Brazil where 6.6% were positive among 76 [5]. However in a study done at UCI, 15 (26.8%) of the children had metastases into the CNS and this is higher than what we found in our study [11]. This difference may be explained by the fact that in our study, very few children underwent this assessment yet in Brazil and at UC all children were assessed for possible CNS disease. It would therefore be ideal to assess all children with ALL for CNS metastases in our setting since it contributes to the survival of these children [24].
In our study, failure to achieve remission was associated with reduced 1 year overall survival. Our finding is not different from what has been reported in other studies across the world. For example studies done in Sweden, Korea, Europe, Asia and America, reported that induction failure was associated with reduced survival due to increased chances of relapse and treatment failure [14,25,26]. A children’s oncology group study also found out that end of induction remission is highly prognostic whereby those who do not achieve remission, are at a higher risk of relapse and death [27,28].
The study was limited by missing data especially about CSF analysis for determining metastases into the central nervous system among some of the children whose samples had been taken off. Also, a big number of children did not undergo this assessment since they presented with severe anemia, severe thrombocytopenia and high white blood cell count and thus a lumber puncture at admission was considered not safe in our circumstances. Lack of flow and cytogenetics was also a hinderance to our study. This was a hindrance to the study since it has been reported that CNS disease is associated with high chances of relapse and reduced survival. However, all children who were not assessed were treated as though they had CNS metastases when it was safe enough to do so. Secondly, we had a small sample size and therefore inferential statistics were less precise. Despite these limitations, the strength of our study is that, it was carried out from a regional referral hospital which has a big catchment area. Therefore, participants from different geographical locations and with varying social demographic backgrounds were studied, thus our study findings can be generalized in the different clinical settings.
In conclusion, the clinical presentation of children diagnosed with ALL at our PCU is similar to that reported elsewhere and can easily be mistaken as other infectious diseases, potentially causing diagnostic challenges and delays. The overall survival was low at MRRH compared to developed countries and failure to achieve remission was associated with reduced survival.
We recommend health education to the caretakers so that they can easily recognize the common clinical features among children with suspected acute leukemia so as to seek early medical care. There should also be increased awareness among primary healthcare workers regarding the clinical presentation and recognition of children with acute leukemia in order to help with early identification and treatment initiation for better outcomes.
Children with ALL present with non-specific clinical features that mimic common childhood infections and its outcomes are low at our unit. ALL should form part of the differential diagnosis in children with fever, pallor, bleeding, or leukocytosis, anemia and thrombocytopenia.
The study was funded by the Massachusetts General Hospital Global Health Collaborative with Mbarara University of Science and Technology through the First Mile Project.
None.
The authors declare no competing financial interest.
[Crossref] [Google Scholar]
Citation: Zalwango S, Kalubi P, Ankunda S, Atwiine B (2024) Clinical Profiles and Survival of Children with Acute Lymphoblastic Leukemia in South West Uganda. Qual Prim Care. 32:03.
Copyright: © 2024 Zalwango S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.