- (2012) Volume 13, Issue 4
Vassilios S Ramfidis1, Alexios S Strimpakos1, Kostas N Syrigos1 and Muhammad W Saif2*
1Oncology Unit, Third Department of Medicine, University of Athens, Sotiria General Hospital. Athens, Greece.
2Department of Medicine and Cancer Center, Tufts Medical Center. Boston, MA, USA
Despite the enormous advances in clinical research in oncology, the prognosis of pancreatic carcinoma remains poor. Thetherapeutic options in this type of cancer are very limited, with modest results at present. In the 2012 American Society of Clinical Oncology (ASCO) Annual Meeting, four interesting trials on the second line treatment of pancreatic cancer were presented. The first study (Abstract #4017) with a phase II design suggested that maintenance therapy with sunitinib, after a complete course of standard first line treatment, was feasible and effective while the second phase I/II study (Abstract #4034) evaluated the role of trabedersen, an agent that inhibits TGF-β2 expression. Finally, the efficacy and toxicity of lapatinib combined with either FOLFOX (Abstract #e14533) or capecitabine (Abstract #e14569) were examined in the second line setting of pancreatic cancer.
Antineoplastic Agents; Drug Therapy; Pancreatic Neoplasms
FOLFOX: 5-flouorouracil, leucovorin and oxaliplatin
Pancreatic cancer is a lethal disease and its prognosis remains dismal despite the recent advances. Concerning the first line therapy in metastatic pancreatic cancer, gemcitabine, with or without erlotinib, was considered for many years the standard of care [1, 2]. Recent data from a phase III study showed that the combination of 5-fluorouracil, leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) was effective and was therefore added to our standard therapeutic array [3].
Unfortunately, there are no standard options available in the second line setting. On disease progression after gemcitabine treatment, the only established therapeutic choice is the combination of 5-flouorouracil, leucovorin and oxaliplatin (FOLFOX), according to the findings of the Charité Onkologie Clinical (CONKO)- 003 trial [4].
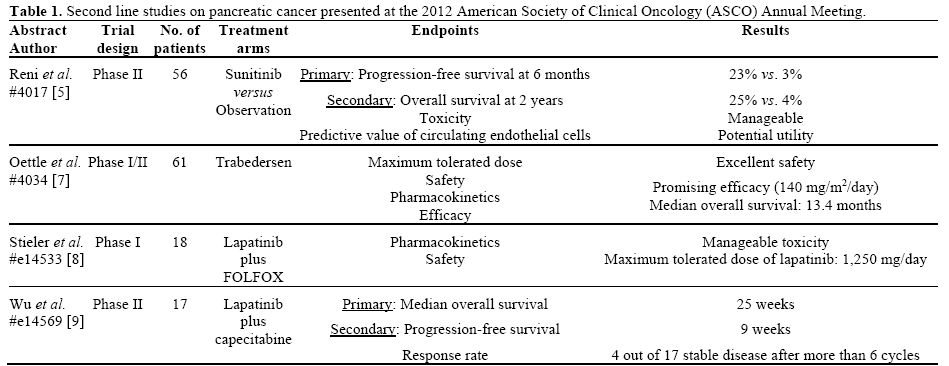
In this year’s ASCO Annual Meeting, four remarkable abstracts regarding maintenance and 2nd line therapy after 1st line treatment of metastatic pancreatic cancer were presented. The purpose of this paper is to present the data and the main findings of these studies as shown collectively in Table 1. The incorporation of the new data on a potential treatment algorithm is illustrated in Figure 1.
Sunitinib as Maintenance Therapy After 1st Line Chemotherapy
Reni et al. (Abstract #4017) conducted an open-label, randomized, multicenter, phase II trial to evaluate the role of sunitinib as maintenance therapy after 1st line therapy in patients with metastatic pancreatic adenocarcinoma. The eligible patients should have achieved disease stabilization after 6 months on chemotherapy and a tumor marker (CA 19-9) with no evidence of rise in excess of 20% from baseline [5]. The fifty-six enrolled patients were equally randomized in two arms. The first arm was the observation group (arm A) while the second one (arm B) received oral sunitinib daily until disease progression or a maximum of 6 months. The primary endpoint was progressionfree survival at 6 months. There was a statistically significant difference of the progression-free survival between the two arms in favor of the active treatment (3% versus 23%; P=0.01). Interestingly, sunitinib was well tolerated with no severe toxicity reported. The majority of patients (76%) in both arms received subsequently a different chemotherapy regimen on progression. The overall survival at 2 years (secondary endpoint) was 4% in arm A versus and 25% in arm B, with a trend to statistical significance (P=0.09). An exploratory analysis within this study tested the predictive role of circulating endothelial cells in patients receiving sunitinib and found that a circulating endothelial cell number greater than 30 was associated with a significantly higher progression-free survival (3.4 months; P=0.01).
Trabedersen (a TGF-β2 Inhibitor) as a New Potential Option in 2nd Line Therapy
Overexpression of the transforming growth factor-beta 2 (TGF-β2) has been suggested as a pivotal factor for malignant progression in pancreatic carcinogenesis [6]. Trabedersen, an inhibitor of TGF-β2, seems to have a role in the management of pancreatic cancer according to a phase I/II study by Oettle et al (Abstract #4034) [7]. A total of 61 patients with solid tumors were recruited and received trabedersen, 37 of which diagnosed with pancreatic carcinoma. The phase I part of the study investigated the maximum tolerated dose, pharmacokinetics, safety and efficacy of this agent. Trabedersen was well tolerated with no severe adverse events, at the dose of 140 mg/m2/day, which was the recommended for the phase II part of this study. The median overall survival of the 37 treated patients with pancreatic cancer was 13.4 months. One patient achieved a complete response, revealing the potential role of this agent in the 2nd line setting of pancreatic cancer management.
Lapatinib in Combination with Fluoropyrimidines as a 2nd Line Treatment of Pancreatic Cancer
Two worth mentioning abstracts, investigating the efficacy of lapatinib (a tyrosine kinase inhibitor against the epidermal growth factor receptor 1 and 2 or else EGFR and HER2) with fluoropyrimidines as second line therapy of pancreatic cancer, were selected for online publication (e-abstracts) at the 2012 ASCO Annual Meeting. In the first abstract, related to a phase I trial, lapatinib was combined with oxaliplatin, folinic acid and 5-fluorouracil (OFF regimen) after gemcitabine failure (Abstract #e14533) [8]. The lapatinib dose ranged from the dose level of 1,000 mg to 1,250 mg and 1,500 mg. The treatment was tolerated with acceptable toxicities apart from the dose level 1,500 mg where one patient developed grade 4 diarrhea with neutropenic enterocolitis and a second one grade 3 diarrhea.
The authors of the second study (Abstract #e14569) examined the activity of lapatinib with capecitabine in an open-label single arm phase II study [9]. The seventeen patients treated with this combination received a median number of 3 cycles (range: 1-22) and showed a median progression-free survival of 9 weeks (95% confidence interval: 7.1-18.9 weeks) and a median overall survival of 25 weeks (95% confidence interval: 11-34 weeks). The most common grade 3 toxicities included nausea (n=3), vomiting (n=3) and diarrhea (n=2).
Despite the enormous advances in clinical research in oncology the prognosis of pancreatic cancer patients remains poor. The therapeutic options in this type of cancer are still limited. New treatment strategies that have been recently developed, such as FOLFIRINOX regimen in the 1st line treatment, might have demonstrated superior results than gemcitabine but should be still interpreted with caution because of its potential toxicity in an incurable disease. Therefore, there is an unmet need of better strategies and new agents in the management of advanced pancreatic cancer.
In this year’s ASCO Annual Meeting, the concept of maintenance therapy with sunitinib after 1st line chemotherapy was presented. This is the first maintenance therapy for pancreatic cancer and the promising results reported by the authors need further confirmation in larger studies. Similarly, the TGF-β2 inhibitor trabedersen demonstrated both good safety profile and evidence of clinical efficacy. These promising results will certainly need validation and in this context a randomized study of trabedersen in 2nd line metastatic pancreatic carcinoma is currently in progress. Finally, the feasibility of the combination of lapatinib with FOLFOX or capecitabine in the 2nd line setting was suggested above, but the clinical activity was somehow modest based on the limited data that are available in the electronic abstracts. More information will be obtained when these studies are published in full.
In conclusion, the main findings on the second line or maintenance therapy from the 2012 ASCO Annual Meeting trials presented above allow optimism and set the platform of new prospective and randomized trials that will confirm the above data.
The authors have no potential conflicts of interest