- (2000) Volume 1, Issue 3
Raffaele Pezzilli1*, Antonio Maria Morselli-Labate2, Anna Rita Barbieri3, Livia Platè3
1Emergency Department, 2Department of Internal Medicine and Gastroenterology, 3Central Laboratory, Sant'Orsola Hospital, University of Bologna. Bologna, Italy
Received March 28th, 2000 Accepted June 27th, 2000
Objective To assess the sensitivity and specificity of the serum carboxypeptidase B activation peptide in diagnosing and determining the severity of acute pancreatitis.
Patients Twenty consecutive patients with acute pancreatitis were studied on admission to the Emergency Room: 11 patients had mild pancreatitis, and 9 patients, severe pancreatitis. Twenty consecutive patients with non-pancreatic acute abdomen and 20 healthy subjects were also studied. Main outcome measures Serum carboxypeptidase B activation peptide was determined using radioimmunoassay.
Results Nineteen of the 20 patients with acute pancreatitis (95.0%) had serum carboxypeptidase B activation peptide concentrations above the upper reference limit, whereas 1 of the 20 patients with nonpancreatic acute abdomen (5.0%) and none of the healthy subjects had serum levels of this protein above the upper reference limit. The serum carboxypeptidase B activation peptide concentrations of patients with severe acute pancreatitis were significantly higher than those of patients with mild acute pancreatitis on the 2nd (P=0.044) and 3rd days (P=0.028) of the study. The overall sensitivity and specificity of carboxypeptidase B activation peptide in assessing the severity of acute pancreatitis were 84.6% and 59.4%, respectively.
Conclusions Serum carboxypeptidase B activation peptide may be used simultaneously both to diagnosis and assess the severity of acute pancreatitis on admission to the Emergency Room.
Amylases; C-Reactive Protein; Carboxypeptidases; Lipase; Pancreatitis; ROC Curve
CAPAP: carboxypeptidase B activation peptide; CRP: C-reactive protein; ROC: receiver operating characteristic
The established management of severe pancreatitis includes aggressive fluid replacement, oxygen supplementation as required, and full intensive care support of any filling organ or system. Early identification of severely ill patients is helpful in order to insure both rapid and appropriate treatment; endoscopic sphincterotomy has become more widely applied for the management of severe gallstone-induced acute pancreatitis [1, 2] and other specific therapies are available or being developed [3-5]. The earlier these treatments are applied, the more effective they will be in the prevention of complications. The need exists for an early objective measure of severity, to select for treatment only those patients who could benefit from it, thus minimizing the numbers of patients treated unnecessarily.
C-reactive protein (CRP), is the most widely used index to assess the severity of acute pancreatitis, however the differentiation between mild and severe disease using this marker is good only 48 hours after the onset of the pain [6, 7]. Appelros et al. [8] have recently shown that the activation peptide of carboxypeptidase B (CAPAP), a 95 amino acid peptide, is able to establish early on the severity of acute pancreatitis. However, these authors [8] assessed the severity of acute pancreatitis according to the serum levels of CRP.
The aims of this study were to assess the sensitivity and specificity of CAPAP in the diagnosis of acute pancreatitis, to evaluate the sensitivity and specificity of this marker in the assessment of the severity of acute pancreatitis according to the Atlanta criteria [9], and to compare the accuracy of this protein in the diagnosis and in the assessment of the severity of acute pancreatitis with that of serum amylase, lipase and CRP.
Subjects
Twenty consecutive patients with acute pancreatitis (14 males, 6 females; average age 67.4 years; range 35-90 years) were studied on admission to the Emergency Room. Eighteen patients were admitted at the onset of the disease and the remaining 2 on the 2nd day after the onset of pain. The diagnosis was based on a history of prolonged upper abdominal pain and the presence of edema or necrosis at abdominal ultrasonography and/or contrast-enhanced computed tomography.
The pancreatitis was of biliary origin in 18 patients which was as suggested by the laboratory data (increased serum levels of conjugated bilirubin, ALT, AST, GGT, alkaline phosphatase), and/or by the history of gallstones and/or by the presence of a dilated common bile duct and biliary sludge and/or stones at ultrasonography and/or contrast enhanced computed tomography and/or endoscopic retrograde cholangiography. Eight patients underwent endoscopic retrograde cholangiography and endoscopic sphincterotomy: stones or sludge in the common bile duct were found in 7 patients and in the remaining one patient, the appearance of the papilla of Vater indicated recent stone passage. The remaining 10 patients underwent surgery for gallstones.
The pancreatitis was due to alcohol abuse in 2 patients (mean daily pure alcohol intake greater than 80 g).
According to the Atlanta criteria [9], 11 patients had mild pancreatitis, and 9 patients had the severe form of the disease
The major complications observed in the patients with severe acute pancreatitis and their frequencies are listed in Table 1. All patients were initially treated conservatively. One of the 9 patients with severe pancreatitis died of cardiac and pulmonary failure on the 5th day after the onset of pain.
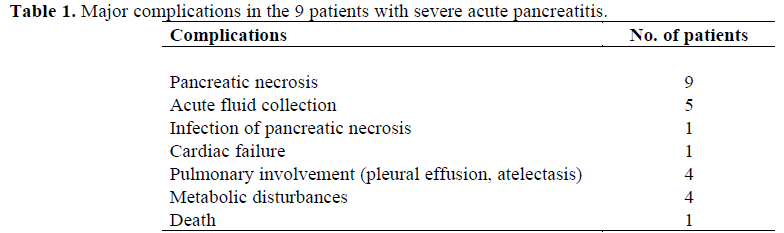
Twenty consecutive patients with nonpancreatic abdominal pain (11 males, 9 females, mean age 57.4 years, range 25-88 years) were also studied. The final diagnoses are listed in Table 2.
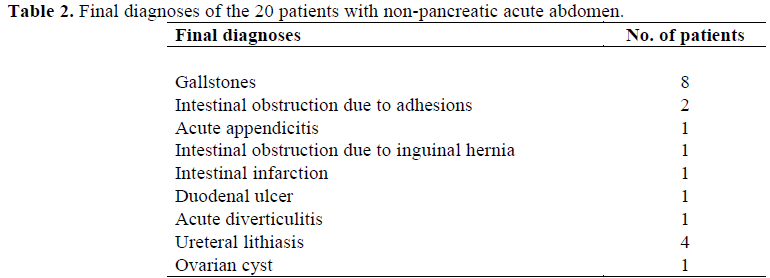
Twenty healthy subjects (12 males, 8 females, mean age 59.6 years, range 20-96 years), recruited from blood donors and subjects who underwent routine medical check-ups, were also studied. All these subjects were in good general health, were not taking any medication and had no signs of disease.
Analytical Methods
After they had given informed consent, blood samples were taken from all study participants upon admission. In the acute pancreatitis patients, blood samples were also collected until the third day after the onset of pain. All samples were frozen immediately after collection and stored at –20 °C until analysis.
Serum CAPAP concentrations were measured using a radioimmunoassay method (CAPAP RIA kit , Euro-Diagnostica, Malmö, Sweden). The kit is based on a competitive radioimmunoassay using antibodies against human CAPAP. CAPAP both in standards and in samples compete with 125I-labelled CAPAP in binding to the antibodies. The 125I-CAPAP binds to the antibodies in an inverse proportion to the concentrations of CAPAP both in standards and samples. Antibodybound 125I-CAPAP is separated from the unbound fraction using the double antibody solid phase technique. The radioactivity of the pellets is then measured. For this assay, the lower limit of detection is about 0.63 nmol/L and the range of the standard curve is comprised between 0 and 20 nmol/L. The upper reference limit is 0.8 nmol/L as reported in the kit information file [10].
Serum amylase concentrations were determined using an enzymatic assay (Amyl, Boehringer Mannheim Systems, Mannheim, Germany) [11]. For this assay, the lower limit of detection is about 2 U/L and the range of the standard curve is comprised between 0 and 1,500 U/L. The reference range of serum amylase, previously established in our laboratory, is 0-220 U/L.
Serum lipase was determined using a turbidimetric technique (Lip, Boehringer Mannheim Systems, Mannheim, Germany) [11]. For this assay, the lower limit of detection is about 5 U/L and the range of the standard curve is comprised between 0 and 1,200 U/L. The reference range of serum lipase, previously established in our laboratory, is 0-270 U/L.
Serum CRP was determined using a nephelometric technique (CRP, Beckman Inc., Brea, CA, USA; normal range 0-0.8 mg/dL) [6]. The lower limit of detection was 0.1 mg/dL and the standard curve ranged from 0.1 to 12 mg/dL; the average percent recovery was 103%.
The within-run precision for the four markers studied were as follows: 8.4% for CAPAP assay, 1.1% for amylase assay, 3.3% for serum lipase and 3% for serum CRP.
The study protocol was approved by the Emergency Department of the S. Orsola Hospital of Bologna, Italy, where the subjects were referred. The study was performed according to the principles of the Declaration of Helsinki for research in human subjects, and all the subjects gave their informed consent.
Age and sex were compared among the three groups of individuals by means of the Kruskal- Wallis and the chi-squared tests, whereas the enzymes were compared among groups using the Mann-Whitney U-test and the Yatescorrected chi-squared test. The sensitivities and specificities of serum CAPAP, amylase, lipase and CRP were compared by means of the Mc-Nemar test [12].
Receiver operating characteristic (ROC) curves, and the respective areas under the curves, were calculated for each marker to provide more accurate information about the accuracy of serum CAPAP and CRP in order to distinguish severe acute pancreatitis from the mild form of the disease [13]. Nonparametric estimates of the areas under the ROC curves and the respective standard errors were applied [14]. A procedure involving paired data was applied to compare areas under the ROC curves [15]. The best cut-off values were chosen as the values that maximized the likelihood ratio (LR) obtained using the following formula:
LR = (probability of true positive + probability of true negative) / (probability of false positive + probability of false negative).
The best cut-off values have been reported as the ranges in which the diagnostic performance of the method did not change [16]. Using these cut-off values, the sensitivity and the specificity in establishing the severity of acute pancreatitis were calculated for serum CAPAP and CRP. The exact 95% confidence intervals (CI) of these proportions were also evaluated [17].
To evaluate the capability of CAPAP in simultaneously establishing the diagnosis and the severity of acute pancreatitis, discriminant analysis [18] was applied to the three groups of patients (acute abdomen, mild acute pancreatitis, severe acute pancreatitis). Discriminant analysis was also applied taking into account both the association of serum amylase with CRP and the association of serum lipase with CRP.
All statistical evaluations were performed running the SPSS/PC+ statistical package on a personal computer [19]. A two-tailed P value less than 0.05 was used to define statistical significance.
Among the patients with acute pancreatitis, those with non-pancreatic acute abdomen, and healthy subjects, there were no significant differences in age (P=0.140) and sex (P=0.611).
Figure 1 shows the individual serum values of CAPAP, amylase, lipase, and CRP on admission to the Emergency Room. Patients with acute pancreatitis had serum concentrations of CAPAP, amylase, and lipase significantly higher (P<0.001) than both those of patients with non-pancreatic acute abdomen and healthy subjects. Serum concentration of these three enzymes were not significantly different between patients with non-pancreatic acute abdomen and healthy subjects. Serum CRP concentrations in healthy subjects were significantly lower than those in patients with acute pancreatitis (P=0.002) and patients with non-pancreatic acute abdomen (P<0.001), whereas no significant differences were found between patients with acute pancreatitis and those with non-pancreatic acute abdomen.
Figure 1. Individual values of serum CAPAP, amylase, lipase and C-reactive protein (CRP) in patients with acute
pancreatitis, in those with non-pancreatic acute abdomen and in healthy subjects. The horizontal solid lines indicate
the upper reference limit of each protein. Eighteen patients with non-pancreatic acute abdomen and 20 healthy
subjects had no detectable serum concentrations of CAPAP (less than 0.63 nmol/L).
a P<0.001 vs. patients with acute pancreatitis
b P<0.01 vs. patients with acute pancreatitis
c P<0.001 vs. patients with non-pancreatic acute abdomen
Nineteen of the 20 patients with acute pancreatitis (95.0%; 95% CI: 75.1-99.9%) had serum CAPAP concentrations above the upper reference limit, whereas 1 of the 20 patients with non-pancreatic acute abdomen (5.0%; 95% CI: 0.1-24.9%) and none of the healthy subjects had serum levels of this protein above the upper reference limit (Table 3). The percentages of the abnormal results of serum CAPAP in patients with acute pancreatitis were significantly higher (P<0.001) than those in patients with nonpancreatic acute abdomen and in healthy subjects, while no significant differences (P=1.000) were found between patients with non-pancreatic acute abdomen and healthy subjects.
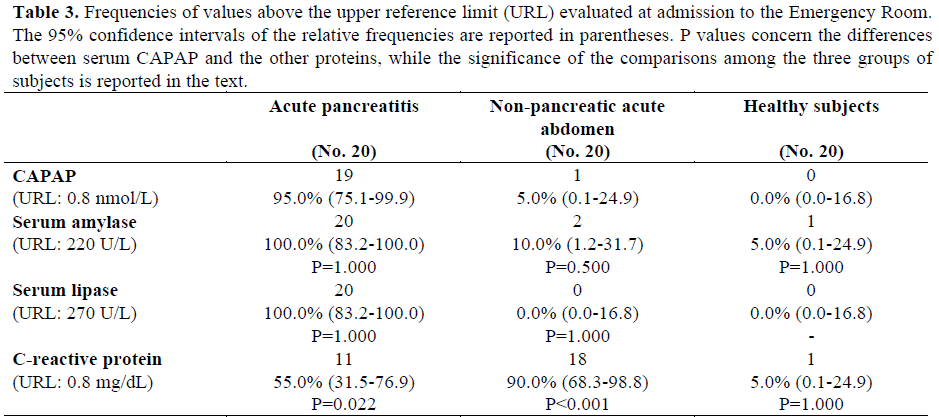
The percentages of the abnormal values of serum amylase, serum lipase, and CRP are reported in Table 3. For serum amylase and lipase, the frequencies of values above the upper reference limit were significantly higher (P<0.001) in patients with acute pancreatitis as compared to those with non-pancreatic acute abdomen and healthy subjects, while no significant differences were found between patients with non-pancreatic acute abdomen and healthy subjects. Regarding serum CRP, the frequency of abnormal results was significantly higher in patients with acute pancreatitis as compared to those with nonpancreatic acute abdomen (P=0.003) and healthy subjects (P=0.002), and in those with non-pancreatic acute abdomen as compared with healthy subjects (P<0.001).
In patients with acute pancreatitis, in those with non-pancreatic acute abdomen and in healthy subjects, the frequencies of the abnormal results of serum CAPAP were similar to those of serum amylase and serum lipase, while in patients with acute pancreatitis and in those with non-pancreatic acute abdomen the frequencies of the abnormal results of serum CAPAP were significantly different from those of serum CRP (P=0.022 regarding patients with acute pancreatitis and P<0.001 regarding patients with nonpancreatic acute abdomen).
Regarding the time course of serum CAPAP, serum amylase and serum lipase, the concentrations of these proteins progressively decreased from the first to the third day of the study (Figure 2), whereas serum concentrations of CRP on the first day of the illness were significantly lower (P<0.001) than those of the second and the third day of the study (Figure 2).
Figure 2. Box and whisker plots of serum CAPAP, amylase, lipase and C-reactive protein (CRP) in patients with
acute pancreatitis during the study period. The boxes represent the interquartile ranges and the lines inside the boxes
represent the median values. The lines emanating from each box (the whiskers) extend to the smallest and largest
observations. The smallest observation and the low interquartile limit of CAPAP are belove the minimum detectable
concentration (less than 0.63 nmol/L).
a P<0.01 vs. the first day of acute pancreatitis
b P<0.01 vs. the second day of acute pancreatitis
The time course of serum CAPAP, amylase, lipase and CRP concentrations in patients with severe acute pancreatitis and in those with the mild form of the disease is reported in Figure 3. CAPAP serum concentrations of patients with severe acute pancreatitis were significantly higher than those of patients with mild acute pancreatitis on the 2nd (P=0.044) and on the 3rd day (P=0.028) of the study, whereas CRP serum concentrations of patients with severe acute pancreatitis were significantly higher than those of patients with mild acute pancreatitis only on the 3rd day of the study (P=0.014). During the three days of the study, serum amylase and lipase concentrations of patients with severe acute pancreatitis were never significantly different from those of patients with mild acute pancreatitis.
Figure 3. Individual values of serum CAPAP, amylase, lipase and C-reactive protein (CRP) in patients with severe and mild acute pancreatitis during the study period. P values concern the difference between severe and mild acute pancreatitis. Twelve serum samples had no detectable CAPAP concentrations (less than 0.63 nmol/L).
In Figure 4, the ROC curves of serum CAPAP and CRP in distinguishing severe from mild acute pancreatitis are reported. The areas under the ROC curves of CAPAP (0.735±0.067) were not significantly different (P=0.890) from those of CRP (0.724±0.068). The ROC curves were not applied to amylase and lipase since no significant differences in their serum concentrations were observed in mild as compared to severe acute pancreatitis.
Figure 4. Receiver operating characteristic (ROC) curves of serum CAPAP and C-reactive protein (CRP) in discriminating between patients with severe acute pancreatitis and mild acute pancreatitis. The values (± standard errors) of the areas under the ROC curves (AUC) and the values of the best cut-off limits are also reported. The optimal operating points relative to the best cut-off values are highlighted on the ROC curves.
In Table 4, the sensitivities and the specificities of serum CAPAP and CRP in establishing the severity of acute pancreatitis are reported. These values were evaluated considering the best cut-off identified by means of the ROC curves (1.14-1.19 nmol/L for serum CAPAP and 1.2-1.4 mg/dL for serum CRP). In particular, the overall sensitivity and specificity were 84.6% (95% CI: 65.1-95.6%) and 59.4% (95% CI: 40.6-76.3%) for serum CAPAP and 84.6% (95% CI: 65.1-95.6%) and 56.3% (95% CI: 37.7-73.6%) for serum CRP.
Using discriminant analysis in simultaneously establishing the diagnosis and the severity of acute pancreatitis, CAPAP alone was able to correctly identify the three groups of patients in 75.0% (95% CI: 58.8-87.3%) of the cases: 100.0% (95% CI: 83.2-100.0%) acute abdomen, 54.5% (95% CI: 23.4-83.3%) mild acute pancreatitis, and 44.4% (95% CI: 13.7- 78.8%) severe acute pancreatitis; the association of serum amylase with CRP correctly classified 77.5% (95% CI: 61.6- 89.2%) of cases: 95.0% (95% CI: 75.1- 99.9%) acute abdomen, 54.5% (95% CI: 23.4-83.3%) mild acute pancreatitis, and 66.7% (95% CI: 29.9-92.5%) severe acute pancreatitis; the combination of lipase with CRP correctly classified 70.0% (95% CI: 53.5-83.4%) of cases: 95.0% (95% CI: 75.1- 99.9%) acute abdomen, 45.5% (95% CI: 16.8-76.6%) mild acute pancreatitis, and 44.4% (95% CI: 13.7-78.8%) severe acute pancreatitis.
The ideal marker for acute pancreatitis should have the following characteristics: high sensitivity and specificity for the diagnosis of the disease, the ability to rapidly assess the severity of the illness, not be observerdependent and it must be inexpensive [20]. At present, there are no markers with these characteristics. Appelros et al. [8] have recently reported that serum CAPAP is a marker of the severity of acute pancreatitis. CAPAP is the activation peptide of procarboxypeptidase B, constituted of 81 amino acids. This peptide is not absorbed from the intestine, does not bind to other substances in the blood and is eliminated through glomerular filtration [21]. About 10% of the peptide filtered by the kidney is recovered in the urine and the remaining 90% is probably digested by the tubuli [21]. CAPAP is very stable in human plasma and urine [21].
We found that CAPAP has a high sensitivity and specificity in diagnosing acute pancreatitis (95% for both), similar to those of serum amylase and lipase which are the indexes most widely used for this purpose [22, 23]. All normal subjects and 95% of the patients with non-pancreatic acute abdomen had serum levels of CAPAP below 0.8 nmol/L and these data are similar to those reported by Appelros et al. [8] and by the kit reference range [10]. Only one patient with intestinal infarction had serum concentrations of CAPAP above 0.8 nmol/L; in this patient, the serum concentrations of amylase were above the upper normal limit, whereas the serum concentrations of lipase were within the normal range.
In assessing the severity of acute pancreatitis, CAPAP, at a cut-off ranging from 1.14 to 1.19 nmol/L, showed an overall sensitivity and specificity similar to those of CRP. Furthermore, CAPAP concentrations in patients with severe acute pancreatitis were significantly higher than those in patients with mild acute pancreatitis on the second and third days of the study, whereas CRP discriminates between the severe and the mild forms of the disease later, namely on the third day of the study [6, 7].
Discriminant analysis showed that CAPAP alone was able to correctly classify 75% of the cases; in particular CAPAP simultaneously identify 100% of patients with acute abdomen, 54.5% of patients with mild acute pancreatitis and 44.4% of patients with severe acute pancreatitis. This figure was very similar to the overall correct classification of patients obtained using the association of serum amylase with CRP (77.5%) and the association of lipase with CRP (70.0%).
Regarding the association of lipase with CRP in simultaneously making the diagnosis and assessing the severity of acute pancreatitis, the results obtained in this study were similar to those reported in a previous paper published by our group [24]. For these reasons, if the results obtained with CAPAP are confirmed by further studies based on a larger number of patients, this protein will make the dream of all pancreatologists a reality; for the first time we will have a marker of acute pancreatitis capable of both simultaneously diagnosing and assessing the severity of the disease on the admission to the Emergency Room. However, at present, only a radioimmunoassay method of analysis is available: a procedure neither simple nor rapid to perform; an immunochemical method which can easily and rapidly determine CAPAP in emergency situations will be welcome.