Case Report - (2023) Volume 6, Issue 3
Complicated Cuffed Tunneled Hemodialysis Catheter Insertion in a Cardiac Transplant Patient
Jafar Alsaid*
Department of Community Health Sciences,, Ochsner Medical Center, Jefferson, USA
*Correspondence:
Jafar Alsaid, Department of Community Health Sciences,, Ochsner Medical Center,
Jefferson,
USA,
Tel: 9259974483,
Email:
Received: 18-Aug-2022, Manuscript No. IPJIIR-22-14492;
Editor assigned: 22-Aug-2022, Pre QC No. IPJIIR-22-14492 (PQ);
Reviewed: 06-Sep-2022, QC No. IPJIIR-22-14492;
Revised: 27-Dec-2022, Manuscript No. IPJIIR-22-14492 (R);
Published:
03-Jan-2023, DOI: 10.21767/2471-8564.23.6.32
Abstract
Cardiac transplant patients are growing in number. AKI with the need for hemodialysis is common among these cases, specifically during the initial phase of their medical course. It is reported that up to 64% of these patients develop AKI of which 28% required hemodialysis. One fifth of these patients ends up having ESRD and requires long term renal replacement therapy. Hemodialysis catheter placement in these patients is crucial for the delivery of renal replacement therapy. Guidelines are lacking in such cases on the preferable central vein to use for the hemodialysis catheter in view of the proximity of the catheter to the cardiac graft. Challenges to insert a cuffed tunneled hemodialysis catheter post cardiac transplant are cardiac assisted devices, central venous congestion and anticoagulation regimen. In this case we would like to share a situation encountered during inserting a Lt. Femoral cuffed tunneled HD catheter with the guidewires entering a dilated iliolumbar venous plexus. With real time fluoroscopy and using iodinated contrast the cuffed tunneled catheter was successfully placed. The requirement for guidelines in these patients is important to assist in providing optimal care.
Keywords
Orthotropic cardiac; Internal jugular catheter; Sternotomy incision; Femoral vein
Introduction
Cardiac transplant patients are being encountered nowadays more often during our clinical practice. More than half will develop AKI during their course and 28% will need hemodialysis. The preferable location to place a vascular catheter had not been clarified. Multiple important factors need to be taken in consideration when planning to insert hemodialysis catheters in these patients these includes history of heart failure with venous congestion, anticoagulation regiment, assisted cardiac devises and the possible shearing force induced by the jet of blood through the catheter towards a cardiac graft [1]. We would like to share a difficulty encountered during inserting a femoral cuffed tunneled hemodialysis catheter in a patient post cardiac transplant.
Case Presentation
Mr. W.G who is a 63 years old African American male patient known to have paroxysmal atrial fibrillation, hyperlipidemia, obstructive sleep apnea, CKD IIIb and Congestive heart failure. He underwent orthotropic cardiac transplant. The postoperative course was complicated with respiratory failure that required assisted ventilation. He developed ventilator associated pneumonia and gram-negative sepsis. Tracheostomy and PEG tube were placed. His kidney function started to decline after developed AKI with ATN. His renal function did not improve and eventually he required Hemodialysis. His clinical condition stabilized after few weeks. We received a consult for placing a cuffed tunneled Hemodialysis catheter. Because of the tracheostomy with excessive secretions, large post OP chest Keloid, an open laceration with purulent discharge on the upper site of the sternotomy incision, the femoral vein was elected as the insertion site [2]. Taking in consideration the recent cardiac transplant and the small caliber Internal Jugular veins noticed with a doppler ultrasound. Both femoral veins were patent with proper unidirectional lamellar flow as determined by the doppler study performed before the procedure.
With real time ultrasound and fluoroscopy guidance, the Rt. femoral vein was punctured after skin preparation, sedation and local anesthesia. The guide wire was introduced but there was resistance encountered at 25 cm. The location of the guidewire, as indicated by fluoroscopy, was deviated laterally at the lumbosacral disk L4-L5 level (Figure 1). We were unable to reposition it properly to the IVC despite attempts to retrieve, rotate and slide the guidewire. We did not want to use contrast in view of his clinical condition. The guidewire and the needle were retrieved (Figure 2).
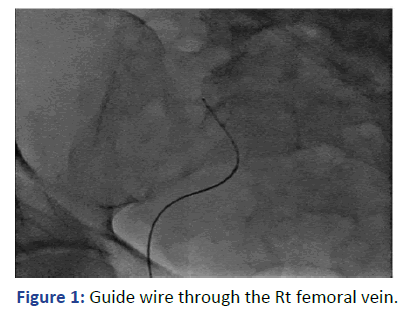
Figure 1: Guide wire through the Rt femoral vein.
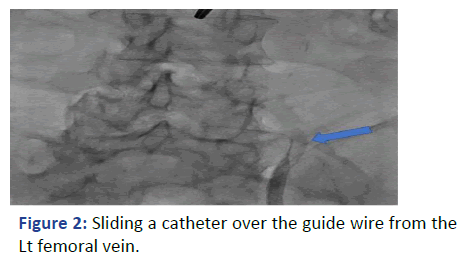
Figure 2: Sliding a catheter over the guide wire from the Lt femoral vein.
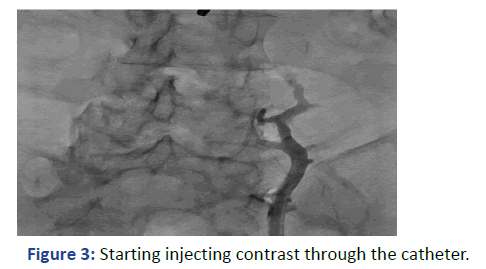
Figure 3: Starting injecting contrast through the catheter.
The Lt. femoral vein was punctured, as an alternative site, with real time ultrasound and under direct fluoroscopy, but we faced the same scenario. The guide wire was located laterally and did not proceed beyond 25 cm. At that point we elected to use Iodine contrast to delineate the venous system anatomy before proceeding further. A venous plexus was illuminated draining from the Lt. common iliac to the lumber region with multiple tributaries (Figures 3 and 4). The catheter was retracted 2 inches and a second contrast dose was injected the Lt (Figure 5). Common iliac and the IVC were clearly visualized this time [3]. The total contrast dose was 35 ml. Double hydrophilic guide wires were passed, with some manipulation, through the Lt. Common iliac vein with the tips at the IVC.
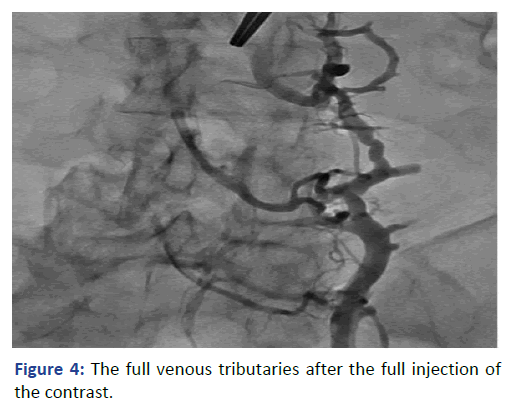
Figure 4: The full venous tributaries after the full injection of the contrast.
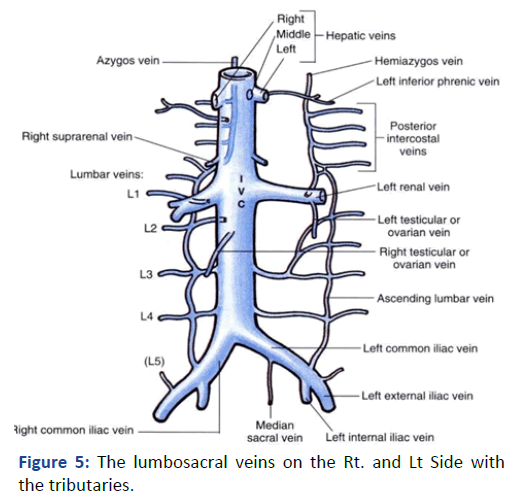
Figure 5: The lumbosacral veins on the Rt. and Lt Side with the tributaries.
The position was confirmed with fluoroscopy using Iodine contrast, (Figures 6-8). A cuffed tunneled catheter was guided with rotation and manipulation under fluoroscopy over the guidewires to the IVC (Figure 9). The position was confirmed with fluoroscopy and the guide wires were retrieved (Figures 10 and 11). The blood flow was checked from both venous and arterial lumen [4]. Heparin lock solution was placed. The catheter was sutured to the skin and the wound was closed. Proper dressing was applied. The patient was stable through the procedure, with no complications.
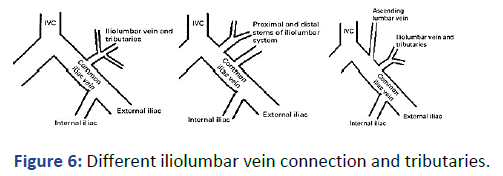
Figure 6: Different iliolumbar vein connection and tributaries.
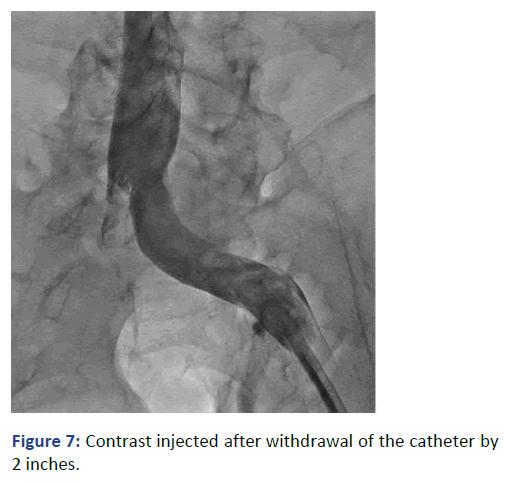
Figure 7: Contrast injected after withdrawal of the catheter by 2 inches.
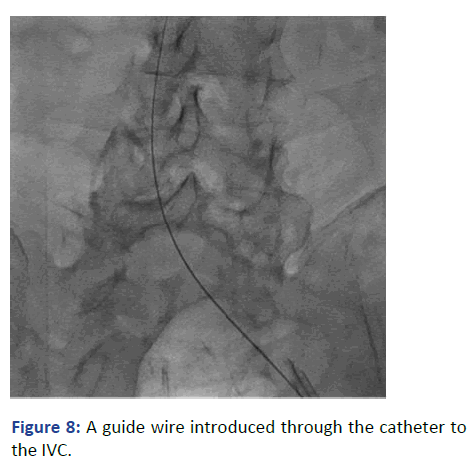
Figure 8: A guide wire introduced through the catheter to the IVC.
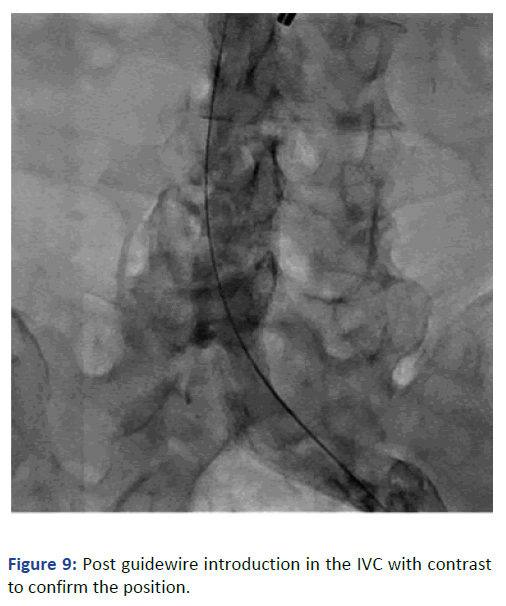
Figure 9: Post guidewire introduction in the IVC with contrast to confirm the position.
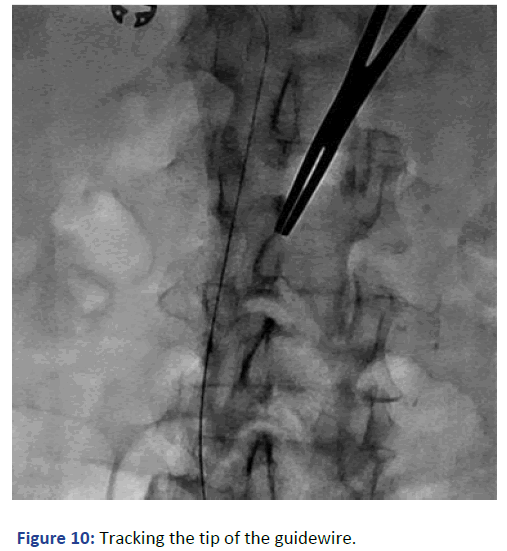
Figure 10: Tracking the tip of the guidewire.
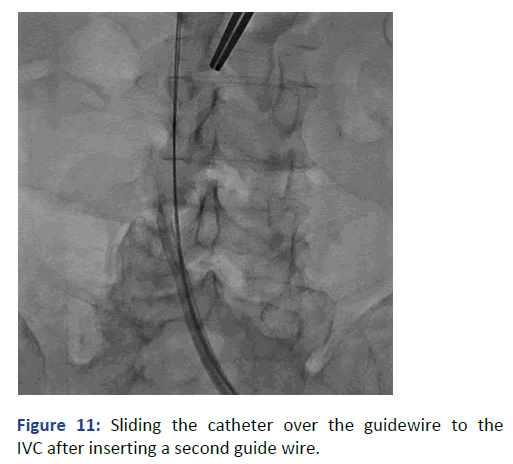
Figure 11: Sliding the catheter over the guidewire to the IVC after inserting a second guide wire.
Discussion
AKI is one of the common complications with patients post cardiac transplant. The incidence is reported to reach 25%-64% according to different publications [5]. Moreover, some of these patients already have CKD with renal impairment. Hemodialysis is required in about 28% of the cases as a supportive process optimizing fluid balance, acid/ base and electrolytes [6]. This would facilitate smooth recovery during the acute phase (Figure 12). It is reported that 19% of the patient post cardiac transplant will end up with ESRD and require renal replacement therapy with 80% continue dialysis and rest undergo renal transplantation.
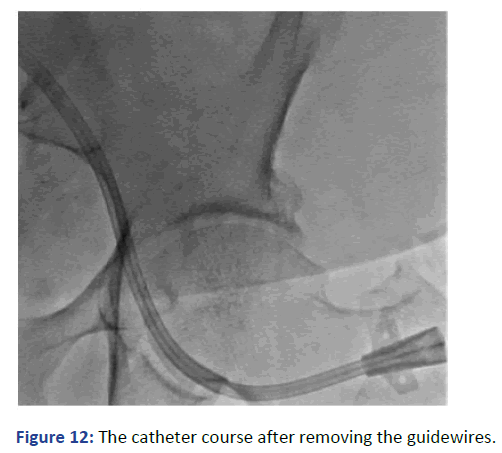
Figure 12: The catheter course after removing the guidewires.
In spite of the increasing numbers of patients with cardiac assisted devises and cardiac transplant we do not have clear guidelines for the preferable vascular access in these patients [7]. Placing an Internal Jugular catheter is recommended in general. But mechanical difficulties and exposing the graftedheart to the high dialysis blood flow, with its sheering force, should be taken in consideration. Whether this would induce inflammation, affect the recovery or contribute to delay healing is still not known. In our case we are addressing difficulty during placing a hemodialysis cuffed tunneled catheter. An enlargement in the iliofemoral venous tributaries with misplaced catheter. This delineates the importance of fluoroscopy as well as using contrast in these cases. In our patient we elected to avoid using the Internal Jugular veins for the reasons mentioned earlier but using the femoral vein was not smooth either (Figure 13). The dilatation in the iliolumbar veinous plexus in patients with heart failure, with assisted cardiac pumps or post cardiac transplant must be kept in mind. Using Fluoroscopy and avoiding bed side femoral access is highly recommended.
Figure 13: The catheter tip after removal of the guidewires.
Iliolumbar venous plexus was reported and illustrated mainly in postmortem studies. It is mentioned that the position of the Iliolumbar veins could be variable. The opening on the iliac veins is reported to be 10.7 mm (+5) with a mean diameter of 3.5 mm (+0.5). Previous publications are reported on postmortem cases. The unique circumstance in our case is that the guidewires were introduced into the venous plexus on both sides. The position of the guidewire on fluoroscopy was identical on both sides. If we did not use the contrast, we would not be able to get the guide wires within the IVC. The total volume of the contrast used was only 35 ml for the whole procedure; this is a small volume to attribute to any deterioration in the renal function. This illustrates the importance of fluoroscopy and contrast in interventional procedure for such patients. Blind approach is highly discouraged.
It is less likely, in our case, that the guidewires entered by chance the iliolumbar venous plexus on both sides. The possible engorged venous tributaries might be the reason behind it. Patients post cardiac transplant, having cardiac assisted devises and with history of congestive heart failure this complication must be kept in mind.
Congenital malposition venous system with a prominent bilateral iliolumbar vein in our patient could not be excluded. However, a wider than usually venous calibers with tributaries due to the increased venous pressure from chronic heart failure or post cardiac assisted device during or prior to the cardiac transplant seems to be a more logical explanation [8]. The importance of using real time fluoroscopy with Iodin contrast could not be emphasized further.
Conclusion
Ultrasound and fluoroscopy guided hemodialysis lines insertion is mandatory to avoid unexpected complications in complicated cases. With expanding number of cardiac transplant patients, the high incidence of AKI and the increasing need to initiate renal replacement therapy, it is highly recommended to have guidelines for temporary vascular access in these patients. It should include preferable anatomical sites and possible complications. Bed side vascular access procedures in such cases are highly discouraged.
References
- Michele D, Jenkins S, Borders S, Wanaga J, Loukas M, et al. (2019) Iliolumbar vein: Anatomy and Surgical importance during lateral transpsoas and oblique approaches to Lumber spine. World Neurosurg. 128:768-772.
[Crossref] [Google Scholar] [PubMed]
- Kiray A, Akcali O, Guvencer M, Terik S, Alici E (2004) Iliolumbar veins have a high frequency of variations. Clin Orthop Relat Res. 425:252-257.
[Crossref] [Google Scholar] [PubMed]
- Gurulingappa Teli C, Kate N, Kothandaraman U (2013) Morphometry of the Iliolumbar artery and the iliolumbar vein and their correlation with the lumbosacral trunk and the obturator nerve. J Clin Diagn Res. 7(3):422-426.
[Crossref] [Google Scholar] [PubMed]
- Florescu M, Sacks A, Um J (2015) Cardiac assist devices and hemodialysis catheter procedures. What do the Nephrologist need to know? Semin Dial. 28(6):670-675.
[Crossref] [Google Scholar] [PubMed]
- Gude E, Andreassean AK, Gullestad L, Grove I, Arora S, et al. (2010) Acute renal failure early after heart transplantation: Risk factors and clinical consequences. Clin Transplant. 24(6): E207-E213.
[Crossref] [Google Scholar] [PubMed]
- Roest S, Hesselink D, Klimczak-Tomanial D, Kardys I, et al. (2020) Incidence of ESRD after heart transplantation and effect of its treatment on survival. ESC Heart Fail. 7(2):533-541.
[Crossref] [Google Scholar] [PubMed]
- Wang T, Lin C, Wei H, Wu M (2020) Long-term outcome and risk factors of renal failure requiring dialysis after heart transplantation: A nationwide cohort study. J Clin Med. 9(8):2455.
[Crossref] [Google Scholar] [PubMed]
- Jocher B, Schilling J, Fischer I, Nakajima T, Wan F, et al. (2021) Acute kidney injury post-heart transplant: An analysis of peri-operative risk factors. Clin Transplant. 35(6):14296.
[Crossref] [Google Scholar] [PubMed]
Citation: Alsaid J (2023) Complicated Cuffed Tunneled Hemodialysis Catheter Insertion in a Cardiac Transplant Patient. J Imaging Interv Radiol. 6:32.
Copyright: © 2023 Alsaid J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.












