Objective: The aim of this study was to perform the forced degradation studies i.e. the effect of
heat, UV light, acid and base on different brands of meloxicam as defined under ICH guideline
Q1A (R2) by using spectrophotometer.
Methodology: The degradation studies were carried out by preparing the standard solution of
200ppm of each brand of meloxicam .The working solutions were prepared from the standard
solution and by the addition of 0.1 N HCl , 0.1 N NaOH and de-ionized water in separate test
tube. The detection of the effect of acid and base were performed by placing the test tubes of
solution of each brand at room temperature and the effect of UV and heat were performed by
leaving the test tubes of solution at 320 nm and 50.C respectively.
Result: The result of this study illustrated that when different brands of meloxicam (A, B and C)
were introduced in 0.1 N NaOH (basic medium) less degradation was observed in brand A and B
while significant degradation was observed in brand C . When different brands of meloxicam (A,
B and C) were introduced in 0.1 N HCl (acidic medium) the two brands B and C showed
significant degradation while brand A showed highly significant degradation. Whereas when the
different brands (A, B and C) were exposed to Ultraviolet light (320 nm) for 30 min, all of three
brands A, B and C showed significant degradation. When different brands (A, B and C) were
exposed to heat (50·C), brand A, B and C showed significant degradation after different time
interval (0, 10, 20, 30, 40, 50 and 60 minutes).
Conclusion: The UV spectroscopy analysis of amount of degraded product is usually preferred
over other methods because of less equipment cost and economical maintenance advantage. It is
easy and rapid method and can be use in routine detection in QC laboratories.
Keywords
Meloxicam, UV spectroscopy, Forced degradation, Different brands and ICH
guideline
Introduction
Meloxicam (figure 1) is chemically
known as 4-hydroxy-2 methyl- ({N-[5-
methyl-2-thiazolyl]-2H-1}, 2-benzothiazine-
3-carboxamide-1, [1-dioxide]). It is a non
steroidal anti-inflammatory drug (NSAID)
which belongs to the oxicam class. It is used
to relieve the symptoms of pyrexia, primary
dysmenorrhea, arthritis, and as an analgesic,
especially where there is an inflammatory
element [1,2]. Meloxicam inhibits the synthesis
of enzyme cyclooxygenase (COX) that is
responsible for converting the arachidonic
acid into prostaglandin H2. This conversion
is the first step in the synthesis of mediators
of inflammatory i.e. prostaglandins.
Meloxicam especially at its low therapeutic
dosage selectively inhibit cyclooxygenase-2
(COX-2) over cyclooxygenase-1 (COX-1)
without affecting platelet aggregation [3-5].
The poor water solubility (0.012 mg/ml) of
meloxicam strongly limited the therapeutic
efficacy [6]. The principal advantage of
meloxicam which permits one daily dosing
is high degree of enterohepatic circulation
and longer half life (15- 20 hrs) aside from
low aqueous solubility [7,8]. The lower dose of
meloxicam (7.5mg/day) is usually prescribed
in gastric disease. Meloxicam is safer
than other NSAID’s though prolonged use
of the drug is associated with gastrointestinal
side effects such as flatulence,
abdominal pain, gastric and duodenal ulcers,
nausea, diarrhea [9,10]. (See figure 1.)
Figure 1: Structure of Meloxicam11
Spectrophotometric technique is
based on measuring the absorption of a
monochromatic light in the near
ultraviolet region (200-380 nm). UV spectro
-photometer can also be use for stress
degradation studies. According to International
Conference of Harmonization
(ICH) guideline the active pharmaceutical
ingredient is focused to various forced
degradation conditions [12] which involve
temperature humidity, acid and base stress
testing, high and low pH variation, photo degradation and time. Thermal with or
without humidity stress testing is performed
by exposing the drug substance to
thermal/humidity conditions in due course
which causes the substance to degrade
forcefully to its main components. UV
degradation is a main problem in numerous
UV-unstable products which are made up of
natural and synthetic polymers as they break
or disintegrate when exposed to constant
sunlight. The attack is dependent on the
degree of exposure as the nonstop exposure
is a major problem than intermittent
exposure. Acid/base forced degradation
testing is used for the evaluation of
degradation of a drug substance by exposure
to basic or acidic medium over time to its
primary degradation products. Acid/base
hydrolysis takes place in labile carbonyl
functional groups for e.g. Alcohols, imines,
imides amides (lactams), esters (lactones),
aryl amines, and carbamates. Forced
degradation is capable of demonstrating that
the preferred technique is stability indicating
and this technique use to identify the raise in
the degradation product and the subsequent
loss of active constituent [13-15]. The aim of the
study is to perform forced degradation
studies of the different brands of meloxicam
under hydrolytic (acidic and basic),
photolytic and thermal stress conditions, as
defined under ICH guideline Q1A (R2) by
using spectrophotometer. It is usually
preferred over other methods because of less
equipment cost and economical maintenance
advantage [17-21].
Experimental
Material and reagents
All the glass materials used in this
research were of Pyrex glass including
stirrer, measuring cylinder, volumetric flask,
pipette and funnel. Glass wares were
initially washed by Chromic acid than rinse with water and finally with DI water or
double distilled water which was freshly
prepared. All the reagents used in the
working were of analytical grade including
Hydrochloric acid (0.1N), Sodium
hydroxide (0.1N) and DI water or double
distilled water. The active use was in the
form of different brands of meloxicam [16].
Instruments
1. Weighing Balance of Pioneer OHAIUS
(Item PA214C),
2. Water Bath with ‘HH-4’ (DGT and
CNST temperature tank.)
3. UV-VIS Spectrophotometer, ‘PG
Instrument’, with a quartz cuvette.
Preparation of 0.1 N sodium hydroxide
and hydrochloric acid
Analytical grade (37% purity and
12N normality) HCl was utilized for the
preparation of 0.1 N HCl which was carried
out by taking small quantity of water in a100
ml volumetric flask and transferring 8.36ml
of hydrochloric acid in a flask and make up
the final volume upto the mark with deionized
(DI) water. The preparation of 0.1 N
NaOH was carried out by weighing 4 grams
of sodium hydroxide and transfer it in 100ml
volumetric flask. Firstly take small portion
of water and dissolve NaOH in it. Finally
make up the volume with de-ionized water
up to the mark of the flask.
Preparation of meloxicam solution of
different brands
Separately weigh each tablet of three
brands of meloxicam. Ground and triturate
the tablets separately with the help of mortar
pestle for each brand to convert them into
powder form. Accurately weighed triturated
powder equivalent to 20 mg of meloxicam
in a tared beaker for each brand i.e. MILT3,
MOBIC MELFAX and dissolve them in
small quantity of DI water for making
primary solutions of meloxicam and shake. The dissolved solutions were transferred
into three different 100ml volumetric flasks.
Lastly make-up the ultimate volume with
de-ionized water to 100 ml for each sample.
By using UV-Visible spectrophotometer, the
absorbance of solutions of each brand of
meloxicam (200ppm) was determined at
wavelength max of 260nm.
Procedure for degradation Studies
The degradation studies were carried
out by determining the effect of heat, UV
acid and base on solution of three brands of
meloxicam .The effect of heat and UV light
on MILT3 (A), MOBIC (B) MELFAX (C)
were determine by transferring 5 ml solution
(200ppm) of each brand A, B and C in the
six different test tubes each of the two test
tubes contain same solution of each brand
correspondingly then add 5 ml of de-ionized
water in all of six test tubes. Now
concentrations of the solution become
100ppm. Three test tubes containing
solution of each brand A, B and C was kept
in water bath at 50.C for 60 mins and
another three test tubes containing solution
of each brand was kept in Ultraviolet light
for 30 mins at 320 nm. The effect of acid
and base were determined by transferring 5
ml solution (200ppm) of each brand A, B
and C in the six different test tubes each of
the two test tubes contained solution of A, B
and C brand correspondingly then add 5 ml
of 0.1 N HCl solution in first set of three test
tubes and 5 ml of 0.1 N NaOH solution in
another set of three test tubes
correspondingly. All the test tubes (12 test
tubes) were left for 30 minutes. UV-Visible
spectrophotometer was used for determining
the absorbance of each solution at
wavelength max of 260 nm.
Result and Discussion
We have conducted study on force
degradation parameters of three different
brands of meloxicam i.e. MILT3 (A), MOBIC (B) MELFAX (C). Their
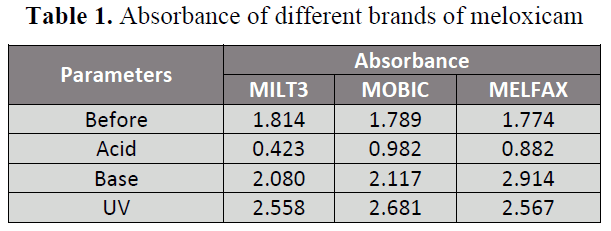
absorbance for degradation parameters
(acid, base and UV) before and after
treatment demonstrated in Table 1 while
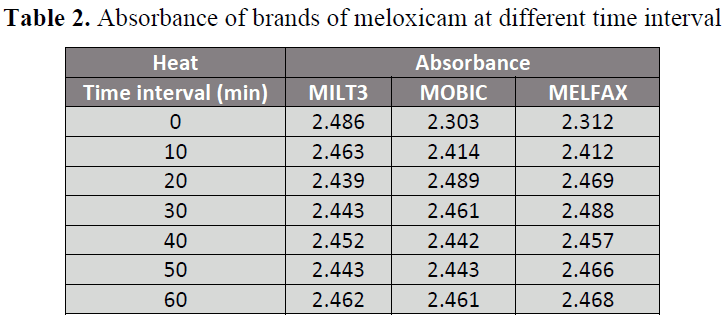
parameter of heat (at different time interval)
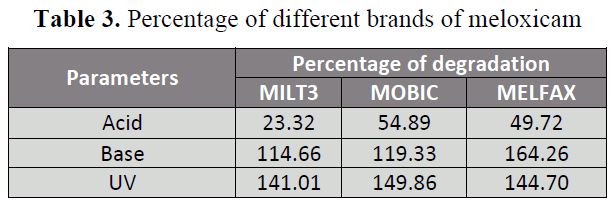
demonstrated in Table 2. The percentage of
degradation of different brands of
meloxicam is shown in Table 3 and 4 and
their graphical representation is shown in Figure 2 and 3.
Table 1: Absorbance of different brands of meloxicam
Table 2: Absorbance of brands of meloxicam at different time interval
Table 3: Percentage of different brands of meloxicam
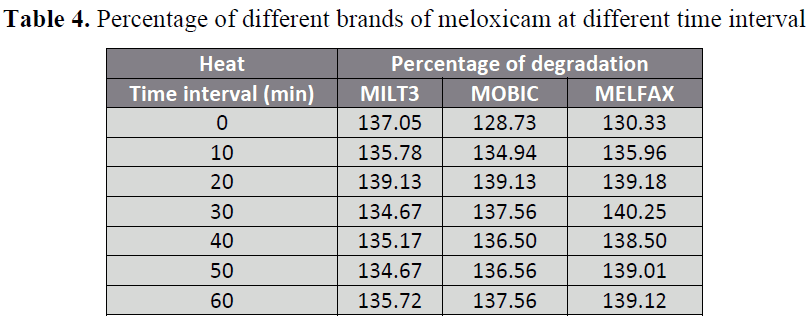
Table 4: Percentage of different brands of meloxicam at different time interval
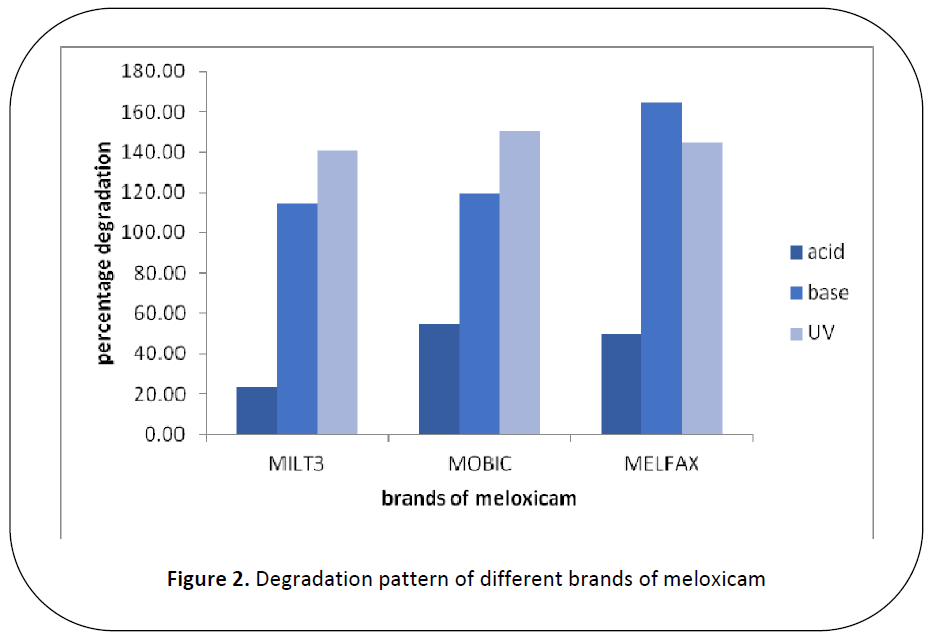
Figure 2: Degradation pattern of different brands of meloxicam
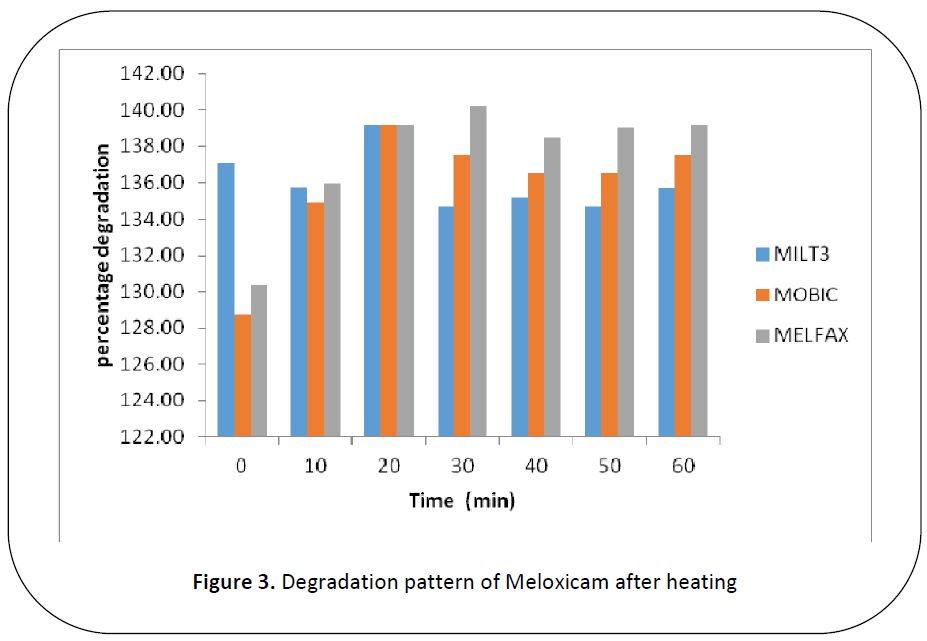
Figure 3: Degradation pattern of Meloxicam after heating
When 3 brands of meloxicam i.e.
MILT3 (A), MOBIC (B) MELFAX (C)
were subjected to 0.1 N NaOH, less changes
in availability were observed in brand A
(114.66%) and B (119.33%) while
significant changes in availability was
observed in brand C (164.26%) with respect
to initial absorbance. When brands of
meloxicam i.e. A, B and C are subjected to
0.1 N HCl, the two brands B and C showed
significant changes in availability i.e.
54.89% and 49.72 % respectively while
brand A showed highly significant changes
in availability 23.32% with respect to initial
absorbance. Similarly when different brands
of meloxicam i.e. A, B and C were exposed
to U.V light, all of the three brands A, B and
C showed significant changes in availability
i.e. 141.01% , 149.86% and 144.70%
respectively . When the 3 brands A, B and C
were subjected to heat (50.C) for 0, 10, 20,
30 ,40, 50 and 60 minutes, brand A shows
slight changes in availability after heating
for 0 min(137.05%), 10 min (135.78%), 20
min (139.13%) , 30 min(134.67%), 40 min
(135.17%) , 50 min (134.67%) and 60 min
(135.72%) . Brand B shows small changes
in availability after heating for 0 min
(128.73%), 10 min (134.94%), 20 min
(139.13%), 30 min (137.56%), 40 min
(136.50%), 50 min (136.56%) and 60 min
(137.56%). Brand C shows changes in
availability which increases gradually with
time after heating for 0 min (130.33%), 10
min (135.96%), 20 min (139.18%), 30 min (140.25%), 40 min (138.50%), 50 min
(139.01%) and 60 min (139.12%).
Conclusion
According to USP, meloxicam tablet
contains not less than (NLT) 90.0 % and not
more than (NMT) 110.0 % of the labeled
amount of (C14H13N3O4S2) meloxicam. The
result of this study concludes that when
chosen brands of meloxicam (A, B and C)
were introduced in 0.1 N NaOH (basic
medium) less degradation was observed in
brand A and B while significant degradation
was observed in brand C . When chosen
brands of meloxicam (A, B and C) were
introduced in the 0.1 N HCl (acidic medium)
the two brands B and C showed significant
degradation while brand A showed highly
significant degradation. Whereas when the
chosen brands (A, B and C) were exposed to
Ultraviolet light (320 nm) for 30 min, all of
the three brands A, B and C showed
significant degradation .When chosen
brands (A, B and C) were exposed to heat
(50·C) for 30 min, brand A, B and C showed
significant degradation after different time
interval (0, 10, 20, 30, 40, 50 and 60
minutes). (See table 1 to 4 and figure 2&3.)
References
- Meineke I, Turck D. Population pharmacokinetics analysis of meloxicam in rheumatoid arthritis patients. Br J Clin Pharmacol 2003; 55 (suppl 1): 32-8.
- Corveleyn. S, Remon JP. Formulation and production of rapidly disintegrating tablets by lyophilization using hydrochlorothiazide as a model drug. Int J Pharm. 1997; 152: 215-225.
- Remon JP, Corveleyn S. Freeze-dried rapidly disintegrating tablets. US patent 6 010 719. January 4, 2000.
- MD Frank Degner, Ralf Sigmund, MD Henning Zeidler. Efficacy and tolerability of meloxicam in an observational, controlled cohort study in patients with rheumatic disease. Clinical Therapeutics; 22(4), Pages 400–410, April 2000.
- Philip Schoenfeld. Gastrointestinal safety profile of meloxicam: a metaanalysis and systematic review of randomized controlled trials. The American Journal of Medicine, 107(6), 54; 13 December 1999.
- Seedher N, Bhatia S. Solubility enhancement of COX-2 inhibitors using various solvent systems. AAPS Pharm Sci Tech 2003; 4: 36-44.
- Heinemann H, Rothe W. Preparation of porous tablets. US patent 3 885 026. May 20, 1975.
- Davies NM, Skojdt NM. Clinical pharmacokinetics of meloxicam: A cyclo oxygenase-2 preferential Non steroidal anti inflammatory drug. Clinical Pharmacokinetics 1999; 36 (suppl 2): 115-126.
- Ellsworth AJ, Witt DM, Dugdale DC, Oliver LM, Mosbay’s 2004 Medical Drug Reference. Elsevier science, Missouri, 2003: 610‐612.
- Knistch A, Hagen E, Munz HD. Production of porous tablets. US patent 4 134 843, January 16, 1979
- Nalini Kanta Sahoo, Madhusmita Sahu, Podilapu Srinivasa Rao, N Sandhya Rani, JNV Indira Devi, and Goutam Ghosh. Validation of assay indicating method development of meloxicam in bulk and some of its tablet dosage forms by RP-HPLC; 3: 95. Springerplus. 2014 doi: 10.1186/2193-1801-3-95.
- ICH. Stability testing of new drug substances and products Q1A (R2), International Conference on Harmonization, IFPMA, Geneva; 2003.
- Food and Drug Administration, HHS (2003) International Conference on Harmonisation; Stability Data Package for Registration Applications in Climatic Zones III and IV; Stability Testing of New Drug Substances and Products; availability. Notice. Fed Regist 68: 65717-65718.
- Kishore kumarhotha et al. (2013) Forced degradation studies: practical approachoverview of regulatory guidance and literature for the drug products and drug substances DOI: 10.7897/2230-8407. 04517.
- R. Nageswara Rao, B. Ramachandra, R. Mastan Vali, S. Satyanarayana Raju. LC–MS/MS studies of ritonavir and its forced degradation products. Journal of Pharmaceutical and Biomedical Analysis Volume 53, Issue 4, Pages 833– 842; 1 December 2010.
- Safila Naveed, Wardha Jawaid, Urooj Bokhari, Hina Qamar. Degradation study of different brands of amlodipine using UV spectrophotometer. Journal of Scientific and Innovative Research 2014; 3(4): 414-418.
- Safila Naveed, Shafiq A, Khan M, Jamal M, Zafar H, et al. (2014) Degradation Study of Available Brands of Metformin in Karachi Using UV Spectrophotometer. J Diabetes Metabolism 5:328. doi:10.4172/2155-6156.1000328,IF 4.39 Index Copernicus Value: 6.17.
- Safila Naveed, Yusra Naseem, Siddiqua Samie, Sheerana Khan, Sana Siddiqui, Bushra and Salma Ramzan (2014) Degradation Study of Five Different Brands of Ciprofloxacin Using UVVisible Spectrophotometer and Their Comparitive study (IRJP; 5(3):189-190 https://www.irjponline.com/admin/php/up loads/2130_pdf.pdf.
- Safila Naveed*, Nimra Waheed and Safeena Nazeer (2014) degradation studies of ampicillin in API and formulations J App Pharm Vol. 6; Issue 3:314-321 https://nebula.wsimg.com/a24 04d9bb84086672affd57eefc846f1?Acces sKeyId=4323AF8BFBC2D34AB0BD& disposition=0&alloworigin=1.
- Safila Naveed*, Hina Qamar, Wardha Jawaid, Urooj Bokhari (2014) Effect of Acid, Base and Time on Different Brands of Glimepiride, Open Access Library Journal, 1.
- Safila Naveed, Nimra waheed and Safeena Nazeer (2014) degradation study of metronidazole in active and different formulation by uv spectroscopy J Bioequiv Availab 2014, 6:4, 124- 127https://dx.doi.org/10.4172/jbb.100019 1.