Esam Mosbah1 and Walaa Awadin2*
1Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
2Departments of Pathology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
*Corresponding Author:
Walaa Awadin
Department of Surgery, Anesthesiology and
Radiology, Faculty of Veterinary Medicine
Mansoura University, Mansoura, Egypt
Tel: +201126797600
E-mail: walaafekriawadin@yahoo.com
Received date: November 30, 2016; Accepted date: December 10, 2016; Published date: March 05, 2017
Citation: Mosbah E, Awadin W. Diagnosis and Management of a Malignant Dermal Melanoma in a Donkey (Equus asinus). J Vet Med Surg. 2016, 1:1. doi: 10.4172/2574-2868.100001
Keywords
Malignant melanoma; Donkey; Histopathology
Abbreviations: AP: Alkaline phosphatase; GGT: Gamma-glutamyl transpeptidase; AST: Aspartate aminotransferase; LDH: Lactate dehydrogenase; Cr: Creatinine; Na: Sodium; RBC: Red blood cell count; PCV: Packed cell volume; Hb: Hemoglobin; MCHC: Mean corpuscular hemoglobin concentration; MCH: Mean corpuscular hemoglobin; MCV: Mean corpuscular volume; WBC: White blood cell count.
Case Description
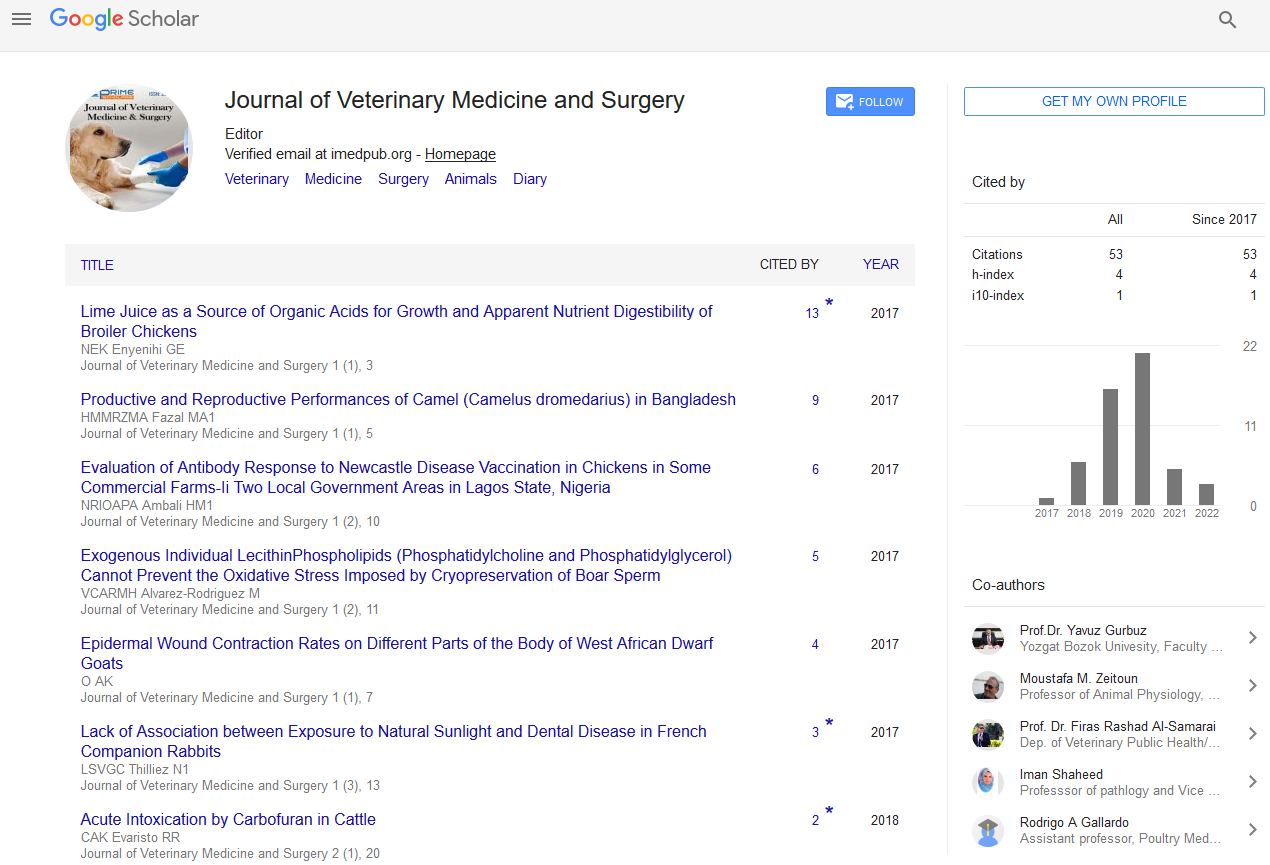
A 2 years old intact male donkey (Equus asinus) was admitted to the Mansoura Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt with two 5 × 10 and 5 × 8 cm diameters, round and firm subcutaneous masses under the tail (Figure 1A).
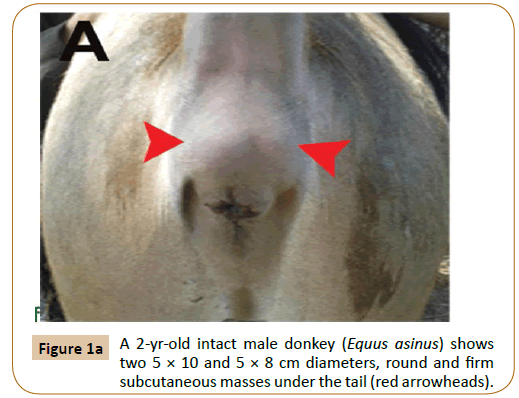
Figure 1a: A 2-yr-old intact male donkey (Equus asinus) shows two 5 × 10 and 5 × 8 cm diameters, round and firm subcutaneous masses under the tail (red arrowheads).
Clinical Findings
Two small lemon sized firm and unpainful masses were distinguished under the tail and above the anal sphincter in a grey colored donkey. Health condition was normal and there was no difficulty during defecation. The owner left the animal 6 months until there was a clear blemish appeared due to increase in the size of the masses. They were hard in consistency and unpainful. The skin covers the swellings was intact. All physical examination findings were within normal limits. A blood sample was collected from the jugular vein for determination of hematologic and plasma biochemical parameters (Table 1).
| |
Before surgery |
Two months after surgery |
Reference range |
| Biochemical parameters |
| AP (IU L-1) |
411 |
425 |
272-605 |
| GGT (UL-1) |
45.82 |
50 |
26.17–86.38 |
| AST (UL-1) |
295.81 |
300 |
173.71–466.07 |
| LDH (IUL-1) |
576.02 |
611.2 |
213.53–1162.81 |
| Urea (mmolL-1) |
7.3 |
7.8 |
5.2-9.4 |
| Cr (µmolL-1) |
127 |
129 |
100.9-178 |
| Total protein (g dL-1) |
8.1 |
8.4 |
7-11 |
| Albumin (g dL-1) |
3.13 |
4.41 |
2.65–3.69 |
| Na (mEqL-1) |
121.50 |
116 |
116.00–132.00 |
| Glucose (mmol L-1) |
1.7 |
2 |
0.46-3.9 |
| Cholesterol (mg dL-1) |
88.41 |
89 |
73.58–124.26 |
| Total bilirubin (mg dL-1) |
0.05 |
0.07 |
0.0-1.0 |
| Hematological parameters |
| RBC (106μL-1) |
5.5 |
5.1 |
4.5-8.2 |
| PCV % |
31.4 |
30.4 |
24-37.5 |
| Hb (g dL-1) |
10.4 |
11.1 |
8.5-12.7 |
| MCHC (g dL-1) |
33.2 |
33.9 |
32.5-35 |
| MCH (pg) |
19 |
20 |
18.2-22.3 |
| MCV (fl) |
52.5 |
60.3 |
53.2-63.6 |
| Platelets (103μL-1) |
228.8 |
220.6 |
42-329 |
| WBC (103μL-1) |
14.1 |
12.4 |
9.8-17.3 |
| Neutrophils % |
46.3 |
46.6 |
33.9-62.4 |
| Lymphocytes |
47.2 |
45.2 |
28.4-59.7 |
| Eosinophils |
4.6 |
4.3 |
1.45-11.1 |
| Monocytes |
1.5 |
1.3 |
1.4-2.9 |
Table 1: Hematology and plasma biochemistry panels.
Treatment and Outcomes
The masses were excised with a combination of sharp and blunt dissection while the donkey was under the effect of tranquilization and epidural anesthesia. The donkey was pre-medicated with intravenous injection of acetylpromazine [Vetranquil 1%, Libourne Cedex, France] at a dose of 0.05 mg/ kg BW. The surgical intervention was performed under epidural injection of a combination of 0.17 mg/kg xylazine HCl [Xylaject, ADWIA, Cairo, Egypt], and 0.22 mg/kg lidocaine HCl [Debocaine, Aldebiky, Egypt] [1].
After aseptic preparation of the area, a linear incision was performed. Both sharp and blunt dissection was performed to isolate the masses. The two primary masses were firm and pigmented. They were dissected from the surrounding subcutaneous and muscular tissues (Figure 1B). The masses were removed en bloc and submitted for histopathology. Bleeding was controlled and the wound was sutured by both simple continuous and simple interrupted sutures using chromic cat gut. The skin was closed by interrupted horizontal mattress suture using prolene. Prophylactic dose of antitetanic serum (3000 iu) was injected subcutaneously as well as 22000 iu/kg penicillin G procaine (Norocillin LA, Norbrook Lab. limited, Newry, Ireland) for 5 successive days post-operation.
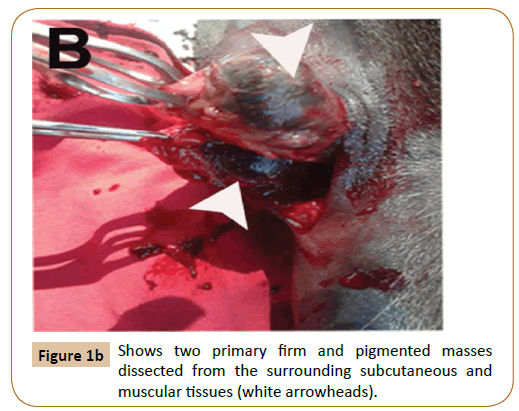
Figure 1b: Shows two primary firm and pigmented masses dissected from the surrounding subcutaneous and muscular tissues (white arrowheads).
Following surgery, the animal was eating and acting normally and showed no signs of disease or postoperative complications.Tissue samples not thicker than 1 cm were fixed for 4-10 days in 10% buffered formalin. Tissue samples were reduced, placed in histology cassettes, and progressively dehydrated in alcohol (70–100%) and xylene. Five micron thick paraffin sections were prepared then stained with H&E. Histopathology of biopsy specimens of the masses showed that the deep dermal, subcutaneous, muscular, adipose tissue and lymph node architecture was variably disrupted by a highly pleomorphic population of heavily pigmented cells arranged in sheets, streams and islands.
These cells had marked anisocytosis and anisokaryosis with single to multiple nuclei (Figure 2A). Mitotic figures were rare. Neoplastic cells were also present within multiple blood vessels (Figure 2B). Moreover, multifocal areas of necrosis and dystrophic calcification were recognized (Figure 2C) associated with thrombosed blood vessels (Figure 2D). Two months after surgery, hematology and plasma biochemistry panels remained within normal limits as shown in Table 1. Healing was achieved without blemish and no recurrence until 5 months follow-up post-surgery as well as no masses appeared in other regions of the body.
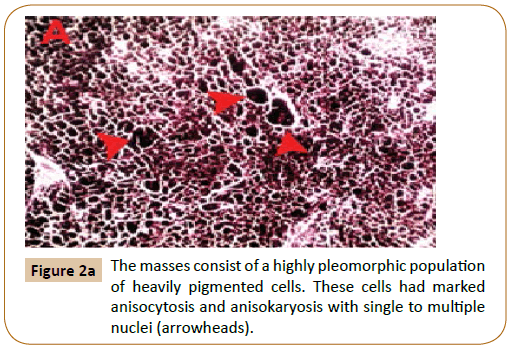
Figure 2a:The masses consist of a highly pleomorphic population of heavily pigmented cells. These cells had marked anisocytosis and anisokaryosis with single to multiple nuclei (arrowheads).

Figure 2b:Neoplastic cells are present within blood vessel (arrowhead).
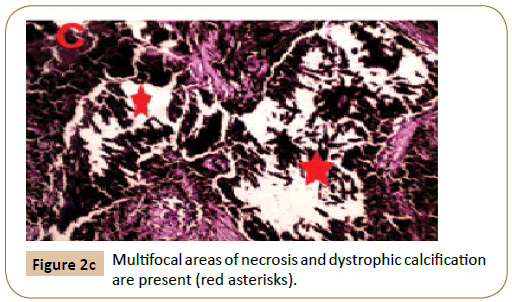
Figure 2c:Multifocal areas of necrosis and dystrophic calcification are present (red asterisks).
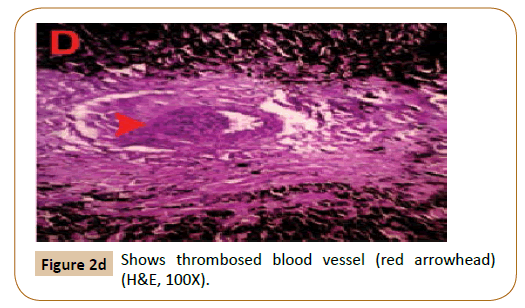
Figure 2d:Shows thrombosed blood vessel (red arrowhead) (H&E, 100X).
Clinical Relevance
Melanocytic tumors develop as a consequence of disturbances in melanin metabolism, which lead to formation of new melanoblasts or increased activity of resident melanoblasts, resulting in overproduction of pigment at a focal area in the dermis [2].
A progression from accumulation of dermal melanocytes at the base of the hair follicles to development of multiple dermal melanocytic tumors was documented in tumor-prone locations. Equine melanoma is one of the most prevalent neoplasia seen, as it was described in 8 to 10 year old grey horses, whereas it was much less common in horses of other colors [3]. Older [4] grey horses have a high prevalence of cutaneous melanocytic tumors that were diagnosed solely according to the gross appearance of the mass [2,5,6].
The most common locations for equine melanoma are the groin, the perineal skin, the lips, and the eyelids [4]. Other common locations include the parotid lymph nodes and salivary glands. In Ref. [4], in one study, more than 79% of the horses had melanoma present in the perineal area, ventral tail, or both sites. In reference [7] another study estimates that up to 80% of grey horses over 15 years old develop melanoma. Melanomas in grey horses are most often non-invasive. In Ref. [4] unlike human malignant melanoma, grey horse melanomas are encapsulated and metastatic progression is prevented or slowed down by factors that are not yet known [8].
Clinical signs depend on the location and size of the tumor. Benign dermal melanomas and malignant dermal melanomatosis look very similar on histopathology and both occur in grey horses in the same predicted regions. In Ref. [8] therefore, neither the location of the tumor nor the histology can help to differentiate between them. One helpful factor that can help to determine the behavior of a tumor is its growth rate. A rapid change in growth rate has been linked to malignant transformation [7].
The etiology of melanomas in horses was not clearly determined.Disturbance of melanin metabolism leading to the development of new melanoblasts, or the increased melanoblasts activity resulting in a central area of too much pigment production in the dermis was suggested to be the most dominant theory in older grey horses. Ultraviolet radiation [sun exposure] may play a role in equine melanoma as in humans. However, the sun exposure doesn't seem to play a significant role in development of equine melanoma, because most melanomas occur in regions not exposed to the sun [9].
In domestic animals, malignancy is determined by tissue site, mitotic index, tumor size, lymphatic invasion, and breed. In Ref. [10] the treatment of choice for patients with melanoma is surgical excision of the mass with at least 2 cm margins. A diagnosis of a malignant dermal melanoma was made in our case based on the size, location of the masses, metastasis into the surrounding lymphoid, muscular, adipose tissues, in addition to, vascular invasion present on histopathology [11].
Metastasis occur secondary to hematogenous and lymphatic spread. Additionally, [2,6,12] in contrast to other species, metastases may arise because of multiple sites of melanocyte proliferation in horses. The [13] regional lymph nodes and lungs were the most common internal organ system affected. Other [12] sites of metastases are the spleen, liver, blood vessels and heart [6,12,14]. 64% of the horses had melanoma masses associated with blood vessels, regional lymph nodes, or both. This Ref. [7] is the first report of a successful surgical excision of a malignant dermal melanoma in an immature donkey. The excision of dermal melanoma was a reasonable treatment option in our case. No regrowth occurred at the surgery site for 5 months following surgery.
Acknowledgements
Departments of Surgery, Anesthesiology, Radiology, and Pathology supported this research; Faculty of Veterinary Medicine, Mansoura University.
References
- Grubb TL, Riebold TW, Huber MJ (1992) Comparison of lidocaine, xylazine, and xylazine/lidocaine for caudal epidural analgesia in horses. Journal of the American Veterinary Medical Association201:1187-1190.
- Johnson PJ(1988) Dermatologic tumors (excluding sarcoids).The Veterinary Clinics of North AmericaEquine practice14: 625-658.
- Levine A (1971) Equine melanotic disease. Tumori 57: 133-168.
- Pilsworth RC, Knottenbelt D (2006) Skin diseases refresher.Equine Veterinary Education18: 228-230.
- Valentine BA (1995) Equine melanocytic tumors: A retrospective study of 53 horses (1988-1991). Journal of Veterinary Internal Medicine9: 291-297.
- White SD, Evans AG, VanMetre DC (2000) Diseases of the skin, melanoma.In: Smith BP (ed.), Large Animal Internal Medicine. St Louis, MO: Mosby,pp: 1225-1226.
- MacGillivray KC, Sweeney RW, Del PF (2002)Metastatic Melanoma in Horses. Journal of Veterinary Internal Medicine16: 452-456.
- Seltenhammer MH, Heere-Ress E, Brandt A (2004) Comparative histopathology of grey-horse-melanoma and human malignant melanoma.Pigment Cell Research 17: 674-681.
- Rashmir RAM, Foy JM, Brashier MK (2006) Cutaneous Tumors in Horses.Western Veterinary Conference. Vin. Com.
- Vail DM, Withrow SJ (2007) Tumors of the skin and subcutaneous tissues. In: Withrow SJ, VailDM.Journal of Zoo and Wildlife Medicine, Withrow & MacEwen’s Small Animal Clinical Oncology. 4th edn. Saunders, St. Louis, Missouri,pp: 375-402.
- Withrow SJ (2000) Surgical oncology. In: Withrow SJ, Vail DM (eds.). Withrow & MacEwen’s Small Animal Clinical Oncology. 4th edn. Saunders St. Louis, Missouri,pp: 157-163.
- Pulley LT, Stannard AA (1990)Melanocytic tumor. In: Moulton JE (ed.), Tumors in Domestic Animals, 3rd edn. Los Angeles (CA): University of California Press,pp: 75-87.
- Schott HC, Major MD, Grant BD, Bayly WM (1990) Melanoma as a cause of spinal cord compression in two horses. Journal of the American Veterinary Medical Association 196: 1820-1822.
- Traver DS, Moore JN, Thornburg LP,Johnson JH Coffman JR (1977) Epidural melanoma causing posterior paresis in a horse. Journal of the American Veterinary Medical Association170: 1400-1403.