Research Article - (2023) Volume 10, Issue 1
Effect of Covid-19 Vaccination on Menstrual Pattern Changes: A Systematic Review
Nathalia Isabella Muskitta1*,
Natan Kevin Partogu Siagian1 and
Amanda Rumondang1,2
1Department of Medicine, University of Indonesia, Indonesia
2Department of Obstetrics and Gynecology, Dr. Cipto Mangunkusumo National Central General Hospital, Indonesia
*Correspondence:
Nathalia Isabella Muskitta,
Department of Medicine, University of Indonesia,
Indonesia,
Email:
Received: 20-Feb-2023, Manuscript No. IPJBR-23-15729;
Editor assigned: 22-Feb-2023, Pre QC No. IPJBR-23-15729 (PQ);
Reviewed: 08-Mar-2023, QC No. IPJBR-23-15729;
Revised: 13-Mar-2023, Manuscript No. IPJBR-23-15729 (R);
Published:
20-Mar-2023, DOI: 10.21767/2394-3718.23.10.06
Abstract
Covid-19 vaccination has proven to be an effective measure to reduce morbidity and mortality. However, some reports have linked Covid-19 vaccination with alterations in menstruation. Considering that menstruation is an important health component for people who menstruate, this information may cause hesitancy to get vaccinated. There are limited systematic reviews regarding the impact of Covid-19 vaccination on changes in menstrual patterns. Therefore, it is necessary to review the available literature on this topic. A systematic review was done in accordance with the PRISMA statement. The literature search was done by hand and on 4 databases: PubMed, EMBASE, ProQuest, and CENTRAL. All found articles were screened based on predetermined criteria. Articles that meet the requirements were critically appraised and analysed. 14 articles consisting of 11 cross-sectional studies and 3 cohorts were found. The outcomes mentioned in the studies include changes in menstrual cycle range, durations, estimated blood quantities, and menstruation symptoms. There are changes in the menstrual pattern after the administration of the Covid-19 vaccination, in the form of irregular menstruation, menorrhagia, worsening of menstruation symptoms, and intermenstrual bleeding. However, these changes only occur temporarily. Therefore, the administration of the Covid-19 vaccination is beneficial for people who menstruate.
Keywords
Covid-19 vaccines; Menstruation disturbances; Menstrual irregularities
Abbreviations
(PICO) Population Intervention Comparison Outcome; (PRISMA)
Preferred Reporting Items for Systematic Reviews and
Meta Analyses; (JBICAT) Joanna Briggs Institute Critical Appraisal
Tool
Introduction
The Covid-19 vaccine prevents the spread of the SARS-CoV-2
virus and reduces the risk of morbidity or mortality [1]. As the
scope of Covid-19 widens, some post-vaccination side effects
have been reported; one of which is menstrual changes [2]. In
the largest surveillance system in the United Kingdom, more
than 36,000 menstrual changes post-vaccination have been reported.
Meanwhile, around the world, there are various results
on menstruation changes post-vaccination [3]. Menstruation is
important in women’s health, especially during the reproductive
period. In this pandemic era, public concern that circumvents
this issue is the effect of Covid-19 vaccination on infertility
regarding its effects on menstruation. Another concern comes
from parents of female adolescents because they think about
the child’s puberty after the Covid-19 vaccination [3]. A deeper understanding of possible menstrual changes post-vaccination
is needed for better counselling before the administration of
the Covid-19 vaccination. However, no systematic review has
discussed the impact of post-covid-19 vaccination on menstruation.
Therefore, the presentation of this systematic review
aims to determine the impact of the Covid-19 vaccination on
menstrual patterns.
Materials and Methods
The clinical question is specified using PICO (Population, Intervention,
Comparison, and Outcome) format (Table 1). This
clinical question is the basis of our systematic review based on
the Preferred Reporting Items for Systematic Reviews and Meta-
Analyses (PRISMA) consensus [4]. We formulated a search
strategy using keywords from the clinical question and its
synonyms. This search strategy is then finely tuned using the
WordFreq and Search Refinery functions from the Systematic
Review Accelerator, Bond University, Australia [5]. Details of
the search strategy are further explored in the appendices.
| Population |
Intervention |
Comparison |
Outcome |
| People who menstruate |
Covid-19 vaccine |
Not vaccinated |
Menstrual changes |
Table 1: Population intervention comparison and outcome (PICO).
The literature search was done on the 16th and 17th of June,
2022 on 4 online databases: PubMed, EMBASE, CENTRAL, and
ProQuest. A manual search was also done based on the referThe literature search was done on the 16th and 17th of June,
2022 on 4 online databases: PubMed, EMBASE, CENTRAL, and
ProQuest. A manual search was also done based on the references
used in the articles. We independently screened all titles
and abstracts found. Studies that are selected by at least 2 researchers
are then reviewed entirely by the eligibility criteria
(Table 2). All conflicts are resolved by discussion. The search
process is reported in a PRISMA flowchart [4].
| Inclusion Criteria |
Exclusion Criteria |
| Hard data (experimental or observational studies) |
Soft data (editorials, news articles) |
| In English or Bahasa |
Full text not available (including conference abstracts) |
| Subjects are otherwise healthy |
Table 2: Eligibility criteria.
Two researchers independently appraised each identified article
using the Joanna Briggs Institute Critical Appraisal Tool (JBI
CAT) [6]. Data extraction was also done by 2 independent researchers
using an online questionnaire that include the study
methods (year, location, and design), subjects (population size
and eligibility criteria), intervention (vaccination status and
type of vaccine), results, and other notable findings. Differing
opinions are resolved by discussion. The conclusion is then
graded according to the GRADE consensus [7].
Results
14 studies fulfill the eligibility criteria (Figure 1). All studies found are observational studies from the last 2 years (2021- 2022). 11 of those studies use a cross-sectional study design [8-18]. The other 3 studies are cohort studies [19-21].
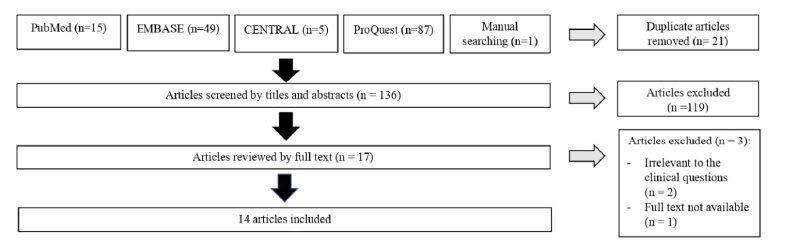
Figure 1: The PRISMA flowchart.
The characteristics of the studies found can be explored in Table 3.
| Article ID (year) |
Population (N) |
Intervention |
Results |
| Alghamdi, et al. (2021)* [8] |
Vaccinated adults, Saudi Arabia. (2,874) |
Pfizer (1,846) vs AstraZeneca (1,028) |
Abnormal menstrual cycle: Pfizer vaccine: 18 (0.69%) subjects (1st dose); 1 (0.22%) subject (2nd dose) vs AstraZeneca vaccine: 7 (0.45%) subjects (1st dose) |
| Almohaya, et al. (2021)* [9] |
Vaccinated adults, Saudi Arabia. (2,302) |
Pfizer vaccine (single arm) |
Lengthening of the menstrual cycle: 8 subjects |
| Anjorin, et al. (2022)* [10] |
Vaccinated adults, Afrika. (455) |
Covid-19 vaccine (single arm) |
Menstrual changes: 5 subjects (0.5%) |
| Araminda, et al. (2022)* [11] |
Vaccinated general population, Indonesia. (298) |
Moderna (138) vs AstraZeneca (160) |
Abnormal menstrual cycle post-vaccination: Moderna 46 subjects (15.4%) vs Astra Zeneca 24 subjects (8%) |
| Dar-Odeh, et al. (2022)* [12] |
Vaccinated doctors and dentists aged 22-71 years old, Saudi Arabia and Jordan. (384) |
Covid-19 vaccine (single arm) |
Menstrual changes: 15 (4.8%) of 314 reproductive-age female subjects. |
| Goldlin, et al. (2021)* [13] |
Vaccinated adults in a teaching hospital. (209) |
Covishield vaccine (single arm) |
Menstrual changes: 1 subject (0.16%) |
| Iguacel, et al. (2021)* [14] |
Vaccinated adults, Spain. (1,446) |
Covid-19 vaccine (single arm) |
Abnormal menstrual cycle: 1.1% subjects (1st dose); 0.5% subjects (2nd dose) |
| Kezia, et al. (2022)* [15] |
Vaccinated adults, Indonesia. (342) |
Sinovac-CoronaVac vaccine (single arm) |
Normal menstrual cycle after 1-3 months follow-up post-vaccination: 273 subjects (79.8%); and |
| 4-6 months follow-up post-vaccination: 294 subjects (85.9%) |
| Lagana, et al. (2022)* [16] |
Vaccinated female adults with a normal menstrual cycle before vaccination, Italy (164) |
1st (9) and 2nd (8) doses of AstraZeneca vs |
Irregular menstrual cycle post-vaccination: 50%-60% (1st dose); 60-70% (2nd dose) |
| Pfizer (133; 133) vs Moderna (19; 14) vs JnJ (3; 0) |
| Muhaidat, et al. (2022)* [17] |
Vaccinated people who menstruate, the Middle East and NorthAfrica Regions. (2,269) |
Covid-19 vaccine (single arm) |
Abnormal menstrual cycle post-vaccination: 66.3% |
| Sa, et al. (2022)* [18] |
Vaccinated general population, U.S.A(6,183) |
mRNA vaccines (Moderna, Pfizer) vs JnJ vaccine |
Dysmenorrhea or abnormal menstrual cycle: Moderna vs Pfizer OR=0.593 (CI=0.561–0.628); Johnson and Johnson vs Pzifer OR=0.992 (CI=0.909–1.083) |
| Edelman, et al. (2022)** [19] |
Vaccinated females aged 18-45 years old with a normal menstrual cycle prior to vaccination, U.S.A. (3,959) |
Covid-19 vaccine (2.403) vs unvaccinated (1.556) |
Lengthening of menstrual cycle post-vaccination in comparison to pre-vaccination: 1st dose 0.71 days (CI=0.47-0.94), 2nd dose 0.91 days (CI=0.63-1.19). |
| Lengthening of menstrual cycle in vaccinated in comparison to unvaccinated population: 1st dose 0.64 days (CI=0.27-1.01), 2nd dose 0.79 days (CI=0.40-1.18). |
| Rogers, et al. (2022)** [20] |
Vaccinated general population aged 18-65 years old, UK (6,855) |
1st (196) and 2nd (3,141) dose of Pfizer vaccine vs 1st (176) and 2nd (2,661) dose of AstraZeneca vs other vaccines (681) |
Menstruation disorder consisted of the abnormal menstrual cycle, increased bleeding, and dysmenorrhea: Pzifer 1st dose 0.6% and 2nd dose 0.4%; AstraZeneca 1st dose 0.2% dan 2nd dose 0.2%. |
| Trogstad, et al. (2021)** [21] |
Female with the normal menstrual cycle and aged 18-30 years old, Norway (8,576) |
1st dose of Covid-19 vaccine (388) vs 2nd dose (5,212) vs unvaccinated (88) |
Increased bleeding after 1st dose RR=1.90 (CI=1.69-2.13), 2nd dose RR=1.84 (CI=1.66-2.03) |
| *Cross-sectional study design; **Cohort study design |
Table 3: Characteristics of included studies.
Results of the critical appraisal using JBI CAT are reported in Table 4 with 3 answer options (Yes (+), Uncertain (?), and No (-)) [6]. 6 studies use online-based self-reported questionnaires; thus we deemed the measurement tools to not be valid (Q3, Q7) [8,9,12-16]. 8 studies ignore possible menstruation-specific confounders due to being a general report of all post-vaccination side effects [8-16]. All 3 cohort studies lacked proper and adequate follow-up periods (Q9, Q10) [19-21].
| Appraisal Tool |
Article ID Criteria |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Q6 |
Q7 |
Q8 |
|
|
|
| JBI CAT for cross-sectional studies |
Alghamdi, et al. [8] |
+ |
+ |
? |
+ |
+ |
- |
- |
+ |
|
|
|
| Almohaya, et al. [9] |
+ |
+ |
? |
+ |
+ |
- |
- |
+ |
|
|
|
| Anjorin, et al. [10] |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
|
|
|
| Araminda, et al. [11] |
+ |
+ |
+ |
+ |
+ |
? |
? |
+ |
|
|
|
| Dar-Odeh, et al. [12] |
+ |
+ |
? |
+ |
+ |
- |
- |
+ |
|
|
|
| Goldlin, et al. [13] |
+ |
+ |
? |
+ |
+ |
- |
- |
+ |
|
|
|
| Iguacel, et al. [14] |
+ |
+ |
? |
+ |
+ |
- |
- |
+ |
|
|
|
| Kezia, et al. [15] |
+ |
+ |
? |
+ |
+ |
- |
- |
+ |
|
|
|
| Lagana, et al. [16] |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
|
|
| Muhaidat, et al. [17] |
+ |
+ |
+ |
+ |
+ |
- |
+ |
+ |
|
|
|
| Sa, et al. [18] |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
Q9 |
Q10 |
Q11 |
| JBI CAT for cohort studies |
Edelman, et al. [19] |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
? |
? |
+ |
| Rogers, et al. [20] |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
? |
+ |
| Trogstad, et al. [21] |
? |
+ |
+ |
- |
- |
? |
+ |
+ |
? |
? |
- |
Table 4: Critical appraisal of included articles using JBI CAT.
Discussion
Studies found in our search had inconsistent definitions of menstrual changes. Studies by Muhaidat et al., Lagana et al., and Rogers et al., use a set of conditions which is considered abnormal [16,17,20]. Lagana et al., defined abnormal menstruation as a menstrual cycle of <25 days or >36 days, menstruation duration of <3 days or >7 days, and an abnormal estimated blood loss in comparison to previous menstruations [16]. In comparison, Muhaidat et al., reported menstrual cramps, menstrual cessation, worsening of premenstrual symptoms and intermenstrual bleeding as its conditions [17]. Other studies either did not prespecify what counts as menstrual changes, or only choose a single condition [9,16,18,21]. These menstrual changes appear variably in different populations. 3 studies mentioned that menstrual disorders are one of the rarer post-vaccination side effects in Africa (0.5%), India (0.16%) and Spain (1st dose: 0.7%, 2nd dose: 0.25%) [10,13,14]. In contrast, menstrual disorders are the second most common side effect (4%, 8%) in female doctors and dentists in Jordan and Saudi Arabia [12]. But, it is consistent that the majority of menstrual changes happen mostly after the first dose (46.7%) in comparison to the second dose (32.4%) or after both doses (20.9%) [17]. One possible cause of the differences between populations is the preferred vaccine used in said population. Post-vaccination menstrual changes were more common after Pfizer-BioNTech than AstraZeneca [8,20]. However, there was no statistically significant difference found [20]. Another study by Sa et al., also found that Pfizer-BioNTech had unfavorable odds ratio for menstrual changes in comparison to Moderna (0.593%, 95% CI=0.561-0.628) and Johnson & Johnson (0.992, CI 0.909-1.083) [18] (Tables 5-8).
| No. |
Query |
Results (n) |
| 1 |
(Covid[Title/Abstract])OR(Covid-19[Title/Abstract])OR(SARSCoV-2[Title/Abstract])OR(BNT162[Title/Abstract])OR(mRNA-1273[Title/Abstract])OR(COVID-19 aAPC[Title/Abstract])OR(INO-4800[Title/Abstract])OR(LV-SMENP-DCCOVID-19 [Title/Abstract])OR(Ad5-nCoV[Title/Abstract])OR(ChAdOx1COVID-19[Title/Abstract])OR(MNA SARS-CoV-2 S1 Subunit[Title/Abstract]) |
251.328 |
| 2 |
(Vaccin*[Title/Abstract])OR(Postvaccin*)OR(Booster Shot[Title/Abstract]) |
379.218 |
| 3 |
1 AND 2 |
33.051 |
| 4 |
(Covid-19 Vaccines[MeSH Terms]) |
12.996 |
| 5 |
3 OR 4 |
34.492 |
| 6 |
(Menstruation Disturbances[MESH Terms])OR(Menstrual Cycle[MESH Terms])OR(Oligomenorrhea[MESH Terms])OR(Amenorrhea[MESH Terms]) |
59.401 |
| 7 |
(Menstrua*[Title/Abstract])OR(Intermenstrua*[Title/Abstract])OR(Premenstrua*[Title/Abstract])OR(Menses[Title/Abstract])OR(Oligomenorrhea[Title/Abstract])OR(Polymenorrhea[Title/Abstract])OR(Amenorrhea[Title/Abstract])OR(Hypomenorrhea[Title/Abstract])OR(Dysmenorrhea[Title/Abstract]) |
73.191 |
| 8 |
6 OR 7 |
96.124 |
| 9 |
5 AND 8 |
15 |
Table 5: PubMed Search Strategy (June 16 2022, 09:54:21 PubMed Time).
| No. |
Query |
Results (n) |
| 1 |
("COVID"):ti,ab,kw OR ("COVID-19"):ti,ab,kw OR ("SARS Co-V"):ti,ab,kw OR ("BNT162"):ti,ab,kw OR ("mRNA-1273"):ti,ab,kw (Word variations have been searched) |
10.387 |
| 2 |
("COVID-19 aAPC"):ti,ab,kw OR ("INO-4800"):ti,ab,kw OR ("LV-SMENP-DC COVID-19"):ti,ab,kw OR ("Ad5-nCoV"):ti,ab,kw OR ("ChAdOx1 COVID-19"):ti,ab,kw (Word variations have been searched) |
24 |
| 3 |
("MRNA SARS-CoV-2 S1 Subunit"):ti,ab,kw (Word variations have been searched) |
0 |
| 4 |
1 OR 2 OR 3 |
10.839 |
| 5 |
("Vaccin*"):ti,ab,kw OR ("Postvaccin*"):ti,ab,kw OR ("Booster Shot"):ti,ab,kw (Word variations have been searched) |
28.097 |
| 6 |
4 AND 5 |
1.412 |
| 7 |
MeSH descriptor: [COVID-19 Vaccines] explode all trees |
147 |
| 8 |
6 OR 7 |
1.412 |
| 9 |
MeSH descriptor: [Menstruation Disturbances] explode all trees |
2.141 |
| 10 |
MeSH descriptor: [Oligomenorrhea] explode all trees |
47 |
| 11 |
MeSH descriptor: [Amenorrhea] explode all trees |
342 |
| 12 |
9 OR 10 OR 11 |
2.141 |
| 13 |
("Menstrua*"):ti,ab,kw OR ("Intermenstrua*"):ti,ab,kw OR ("Premenstrua*"):ti,ab,kw OR ("Menses"):ti,ab,kw OR ("Oligomenorrhea"):ti,ab,kw (Word variations have been searched) |
1.727 |
| 14 |
("Polymenorrhea"):ti,ab,kw OR ("Amenorrhea"):ti,ab,kw OR ("Hypomenorrhea"):ti,ab,kw OR ("Dysmenorrhea"):ti,ab,kw (Word variations have been searched) |
4.864 |
| 15 |
12 OR 13 OR 14 |
7.045 |
| 16 |
8 AND 15 |
5 |
Table 6: CENTRAL Search Strategy (June 17 2022, 05:41:33 PubMed Time).
| No. |
Query |
Results (n) |
| 1 |
((AB,TI("COVID" OR "COVID-19" OR "SARS COV-2" OR "bnt162" OR "mrna-1273" OR "covid-19 aapc" OR "ino-4800" OR "lv-smenp-dc COVID-19" OR "ad5-ncov" OR "chadox1 COVID-19" OR "MRNA SARS-CoV-2 S1 Subunit") AND AB,TI("Vaccin*" OR "Postvaccin*" OR "Booster Shot")) OR MESH("COVID-19 Vaccines")) AND (MESH("Menstruation Disturbances" OR "Menstrual Cycle" OR "Oligomenorrhea" OR "Amenorrhea") OR AB,TI("Menstrua*" OR "Intermenstrua*" OR "Premenstrua*" OR "Menses" OR "Oligomenorrhea" OR "Polymenorrhea" OR "Amenorrhea" OR "Hypomenorrhea" OR "Dysmenorrhea")) |
87 |
Table 7: ProQuest Strategy (June 17 2022, 09:14:02 PubMed Time).
| No. |
Query |
Results (n) |
| 1 |
'covid':ab,ti OR 'covid-19':ab,ti OR 'sars cov-2':ab,ti OR 'bnt162':ab,ti OR 'mrna-1273':ab,ti OR 'covid-19 aapc':ab,ti OR 'ino-4800':ab,ti OR 'lv-smenp-dc covid-19':ab,ti OR 'ad5-ncov':ab,ti OR 'chadox1 covid-19':ab,ti OR 'mna sars-cov-2 s1 subunit':ab,ti |
265.834 |
| 2 |
'vaccin*':ab,ti OR 'postvaccin*':ab,ti OR 'booster shot':ab,ti |
444.94 |
| 3 |
1 AND 2 |
34.167 |
| 4 |
'sars-cov-2 vaccine'/exp |
19.651 |
| 5 |
3 OR 4 |
37.9 |
| 6 |
'menstrua*':ab,ti OR 'intermenstrua*':ab,ti OR 'premenstrua*':ab,ti OR 'menses':ab,ti OR 'oligomenorrhea':ab,ti OR 'polymenorrhea':ab,ti OR 'amenorrhea':ab,ti OR 'hypomenorrhea':ab,ti OR 'dysmenorrhea':ab,ti |
95.121 |
| 7 |
'menstruation disorder'/exp OR 'menstrual cycle'/exp |
193.483 |
| 8 |
6 OR 7 |
217.721 |
| 9 |
5 AND 8 |
60 |
| 10 |
9 AND [embase]/lim |
49 |
Table 8: EMBASE Strategy (June 17 2022, 23:30:01 PubMed Time).
Changes in menstrual cycle duration were found after the 1st dose of AstraZeneca (55.6%), Pfizer-BioNTech (41.4%), Moderna (52.6%), and Johnson & Johnson (66.7%) vaccines [16]. The average menstrual cycle duration significantly increased from 27+6 days (pre-vaccination) to 28.1+10 days (post-vaccination) [17]. This finding is supported by Edelman et al., who also reported significant menstrual cycle duration change in the vaccinated population in comparison to the unvaccinated cohort [19]. Increased menstrual cycle duration can be due to increased menstruation duration (6+0.03 days to 6+0.05 days) or delayed menstruation (0.4%) [9,17]. Menorrhagia is also reported in 78 cases (3.4%) [17]. Relative risk of increased menstrual bleeding was 1.90 (95% CI: 1.69-2.13) and 1.84 (95% CI=1.66-2.03) respectively after the 1st and 2nd dose of vaccination [21]. The risk of increased menstrual bleeding can be due to changes in estimated menstrual bleeding, menstruation frequency, or the aforementioned menstruation duration. Changes in the estimated amount of menstrual blood, which is the most common cause, were found after the first dose of AstraZeneca (66.7%), Pfizer-BioNTech (47.4%), Moderna (52.6%), and Johnson & Johnson (66.7%) [16]. Changes in menstruation frequency are also reported after the first dose of AstraZeneca (66.7%), Pfizer-BioNTech (57.1%), Moderna (47.4%), and Johnson & Johnson (33.3%) vaccines [16]. In Indonesia, Sinovac- CoronaVac vaccine is more extensively used during the earlier vaccination effort. Menstrual changes post-Sinovac-CoronaVac administration happens in 20.2% of patients during the first 3 months [15]. During the next 3 months, the percentage decreases to 14.1% [15]. Results of other vaccines in Indonesia show that irregular menstruation is more likely found patients given Moderna (15.4%) than AstraZeneca (8%) vaccines (p=<0,000) [11].
The mechanism of Covid-19 vaccination altered menstruation is still unknown. However, a hypothesis links this phenomenon to vaccine-induced thrombocytopenia and the immune system that also occurs in various other types of vaccines, such as Measles Mumps Rubella (MMR), hepatitis A, hepatitis B, and others [17]. Thrombocytopenia could explain frequent menstrual bleeding [18]. In addition, the increased menstrual disturbance after the Covid-19 vaccination was also associated with anxiety or stress because the Covid-19 vaccine was still newly discovered [13]. Another hypothesis is the ability of mRNA-based vaccines that were assessed to induce novel immune responses that could affect the hypothalamic-pituitary-ovarian axis [19].
Menstrual changes that occurred post-Covid-19 vaccination have negatively affected the quality of life of more than 50% of women [17]. A slight change in menstruation could be a problem, especially for someone who planning or avoiding pregnancy [19]. However, menstrual changes that occur are generally moderate (54%) and did not require hospitalization [20]. Data showed that 92.3% of women who experience menstrual disorders after the 1st dose of vaccination still continue to get the 2nd dose of vaccination [21]. It is because menstrual disorders that occur are generally temporary and do not affect fertility [12]. Therefore, giving Covid-19 vaccination is still considered to have greater benefits than not clinically significant menstrual changes [19,21]. This study describes various changes in menstruation after Covid-19 vaccination with various types of vaccines, either mRNA or vector-based. This broad discussion topic is supported by an article search strategy designed to be as sensitive as possible. The limitation of this study lies in the lack of articles specifically written to discuss the PICO carried. In addition, the majority of studies found are based on online self-reporting, so there is a risk of memory and representational bias. Based on these considerations, the researchers assess that the synthesis of evidence in this article meets the very low-GRADE criteria [7]. This shows that quality research is still very much needed in this area of science.
Conclusion
Based on the studies that have been described, there are variations
in menstrual changes after the Covid-19 vaccination seen
from the cycle, duration, frequency, quantity of bleeding, and
other complaints related to menstruation. The mechanism behind
this is still unclear, but it is associated with thrombocytopenia,
stress, and effects on the hypothalamic-pituitary-ovarian
axis. Menstrual changes after the Covid-19 vaccination can
affect women’s quality of life, but only temporarily. Therefore,
giving the Covid-19 vaccination is still beneficial for reproductive
women.
Declaration
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Funding
This research received no external funding.
Authors Contribution
Conceptualization: N.I.M., N.K.P.S and A.R
Methodology: N.K.P.S
Investigation: N.I.M., N.K.P.S and A.R
Data curation: N.I.M. and N.K.P.S
Writing-original draft preparation: N.I.M
Writing-review and editing: A.R
Visualization: N.I.M. and N.K.P.S
Supervision: A.R
Project administration: N.I.M
Funding acquisition: A.R
All authors read and approved the final manuscript.
Orcid Details
Nathalia Isabella Muskitta: https://orcid.org/0000-0003-0999-
4227
Natan Kevin Partogu Siagian: https://orcid.org/0000-0003-
3073-5027
Amanda Rumondang: https://orcid.org/0000-0002-7949-4415
Acknowledgement
Not applicable.
Conflict of Interest
The authors declare that they have no conflict of interests.
References
- Padda IS, Parmar M (2023) COVID (SARS-CoV-2) vaccine. StatPearls.
[Crossref] [PubMed]
- Medeiros KS, Costa APF, Sarmento ACA, Freitas CL, Goncalves AK (2022) Side effects of COVID-19 vaccines: A systematic review and meta-analysis protocol of randomised trials. BMJ Open 12(2): e050278.
[Crossref] [Google Scholar] [PubMed]
- Male V (2022) Menstruation and Covid-19 vaccination. BMJ 376: 0142.
[Crossref] [Google Scholar] [PubMed]
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6(7): e1000097.
[Crossref] [Google Scholar] [PubMed]
- Clark J, Glasziou P, Del Mar C, Bannach-Brown A, Stehlik P, et al. (2020) A full systematic review was completed in 2 weeks using automation tools: A case study. J Clin Epidemiol 121: 81-90.
[Crossref] [Google Scholar] [PubMed]
- Critical appraisal tools (2022).
- Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions.
- Alghamdi AN, Alotaibi MI, Alqahtani AS, Alboud DA, Abdel-Moneim AS (2021) BNT162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front Med (Lausanne) 8(760047): 1-10.
[Crossref] [Google Scholar] [PubMed]
- Almohaya AM, Qari F, Zubaidi GA, Alnjajim N, Moustafa K, et al. (2021) Early solicited adverse events following the BNT162b2 mRNA vaccination, a population survey from Saudi Arabia. Prev Med Rep 24(101595): 1-9.
[Crossref] [Google Scholar] [PubMed]
- Anjorin AA, Odetokum IA, Nyandwi JB, Elnadi H, Awiagah KS, et al. (2022) Public health surveillance for adverse events following COVID-19 vaccination in Africa. Vaccines (Basel) 10(4): 546.
[Crossref] [Google Scholar] [PubMed]
- Araminda GN, Ramatillah DL (2022) Evaluation comparison between astrazeneca and moderna vaccine’s side effects and efficacy among Indonesia society based on sociodemography. Int J Appl Pharm 14(2): 37-43.
[Crossref] [Google Scholar]
- Dar-Odeh N, Abu-Hammad O, Qasem F, Jambi S, Alhodhodi A, et al. (2022) Long-term adverse events of three COVID-19 vaccines as reported by vaccinated physicians and dentists, a study from Jordan dan Saudi Arabia. Hum Vaccin Immunother 18(1): 2039017.
[Crossref] [Google Scholar] [PubMed]
- Goldlin TJA, Kalyanaraman S, Ravichandran M, Ramya JE (2021) A pharmacovigilance study of covishield in a tertiary care teaching hospital in Tamil Nadu. J Pharmacol Pharmacother 12: 131-136.
[Crossref] [Google Scholar]
- Iguacel I, Maldonado AL, Ruiz-Cabello AL, Casaus M, Moreno LA, et al. (2021) Association between COVID-19 vaccine side effects and body mass index in Spain. Vaccines (Basel) 9(11): 1321.
[Crossref] [Google Scholar] [PubMed]
- Kezia V, Ramatillah DL (2022) Intensive monitoring of sinovac vaccine for safety and efficacy among Indonesian population. Int J Appl Pharm 14(2): 44-48.
[Crossref] [Google Scholar]
- Lagana AS, Veronesi G, Ghezzi F, Ferrario MM, Cromi A, et al. (2022) Evaluation of menstrual irregularities after COVID-19 vaccination: Results of the MECOVAC survey. Open Med (Wars) 17(1): 475-84.
[Crossref] [Google Scholar] [PubMed]
- Muhaidat N, Alshrouf MA, Azzam MI, Karam AM, Al-Nazer MW, et al. (2022) Menstrual symptoms after COVID-19 vaccine: A cross-sectional investigation in the MENA region. Int J Womens Health 14: 359-404.
[Crossref] [Google Scholar] [PubMed]
- Sa S, Lee CW, Shim SR, Yoo H, Choi J, et al. (2022) The safety of mRNA-1273, BNT1626b2, and JNJ-78436735 COVID-19 vaccines: Safety monitoring for adverse events using real-world data. Vaccines (Basel) 10(2): 320.
[Crossref] [Google Scholar] [PubMed]
- Edelman A, Boniface ER, Benhar E, Han L, Matteson KA, et al. (2022) Association between menstrual cycle length and Coronavirus disease 2019. Obstet Gynecol 139(4): 481-489.
[Crossref] [Google Scholar] [PubMed]
- Rogers A, Rooke E, Morant S, Guthrie G, Doney A, et al. (2022) Adverse events and overall health and well-being after COVID-19 vaccine. BMJ Open 12: e060583.
[Crossref] [Google Scholar]
- Trogstad L, Laake I, Robertson AH, Mjaaland S, Caspersen IH, et al. (2021) Increased occurrence of menstrual disturbances in 18-to-30 year old women after COVID-19 vaccination. SSRN Electronic Journal
[Google Scholar]
Citation: Muskitta NI, Siagian NKP, Rumondang A (2023) Effect of Covid-19 Vaccination on Menstrual Pattern Changes: A Systematic Review. Br J Res. 10:06.
Copyright: © 2023 Muskitta NI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.