- (2014) Volume 15, Issue 4
Yinfeng Shen1, Xiaochuan Deng2, Wenyin Jin1, Chenwei Zhang3, Xingwen Zhang3, Ying Wang3
1Department of Surgery, Hubei Hospital of Chinese Medicine, 2Hubei Hospital of Chinese Medicine, 3Clinical School, Hubei University of Chinese Medicine, Wuhan, P.R. China
Received April 12th, 2014 – Accepted June 13th, 2014
Objective To evaluate the effectiveness of pharmaconutrition-supplemented parenteral nutrition (PN) for severe acute pancreatitis (SAP). Methods A comprehensive search of abstracts was performed in the MEDLINE, OVID, Springer, and Cochrane Library database. Published data of randomized clinical trials (RCTs) comparing the clinically relevant outcomes of pharmaconutrition-supplemented PN versus PN for patients with SAP were analyzed. The analyzed outcome variables included infection, mortality, intensive care unit (ICU) stay, hospital stay, and leukocytes change. Statistical analyses were performed using the Cochrane Collaboration’s RevMan 5.1 software. Results Four RCTs published in 1998 or later were included in this meta-analysis, in which 76 patients with pharmaconutrition-supplemented PN and 77 patients with PN. Pharmaconutrition-supplemented PN showed significantly better results in terms of infection (OR, 0.42; 95% CI,0.20–0.91; P =0.03) and leukocytes change (before treated: mean different, 0.93; 95% CI, 0.21–1.65; P =0.01; after treated: mean different,-0.77; 95% CI, -1.47– -0.08; P =0.03). No significant difference could be found in mortality (OR, 0.30; 95% CI, 0.07–1.19; P =0.09), ICU stay (mean different, -3.65; 95% CI, -9.39–2.10; P =0.21), and hospital stay (mean different, -1.20; 95% CI, -9.89–7.48; P =0.79). ConclusionsThe current meta-analysis indicates that pharmaconutrition-supplemented PN only show advantages in infection and leukocytes change.
Infection; Intensive Care Units; Length of Stay; Leukocyte Count; Meta-Analysis; Mortality; Pancreatitis, Acute Necrotizing; Parenteral Nutrition; Severity of Illness Index
SAP: severe acute pancreatitis; PN: parenteral nutrition; RCT: randomized clinical trial; EN: enteral nutrition
Severe acute pancreatitis (SAP) is a relatively common gastrointestinal emergency, and the patient will experience multiple systemic and/or local complications, such as multiple organ failure, necrosis, abscess, and formation of pancreatic pseudocysts, with a high mortality rate of 10–30% [1, 2]. Despite advances in treatment techniques, the mortality of SAP was stable during the past several decades. How to survive more patients in the first phase which is characterized by systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) [3], the management of SAP remains a challenge for most gastroenterologists. Secondary infections of pancreatic necrosis and subsequent septic complications have emerged as the main risk factor for late death in severe acute pancreatitis [4]. Prophylactic antibiotics cannot reduce infected pancreatic necrosis in patients with SAP [5]. Much focus has been placed on nutritional support.
Nutritional support plays an important role in the management of patients with SAP, expected to decrease morbidity and mortality due to repaired immune function, decreased risk of sepsis. Nutritional support in SAP remains a number of different approaches, including the use of nasogastric feeding and nasojejunal enteral nutrition (EN) [6] and parenteral nutrition (PN) [7] support, being adopted in recent clinical studies [8].
However, PN is also useful for patients suffering from SAP accompanied by nausea and vomiting, [9] which nutrient requirements cannot be met by EN [10]. Support of patients with SAP with PN has been suggested to improve functioning of the gastrointestinal system and the pancreas, preventing exocrine secretions responsible for autodigestion of the pancreas, supporting optimal recovery as well as for life support [1]. PN and pharmaconutrition hastened the recovery of SAP patients, stimulated gastrointestinal motility, and alleviated the degree of systemic inflammatory response syndrome. Pharmaconutrition-supplemented PN is a new kind of PN including glutamine, ω-3 fatty acids, and so on, which can decreases hyperinflammatory response and infectious morbidity rate [11, 12].
The effect of parenteral pharmaconutrition on recovery from SAP has not been thoroughly investigated by metaanalyses. This study aimed to examine the effectiveness of pharmaconutrition-supplemented PN for patients with SAP; we performed an up-to-date meta-analysis to pharmaconutrition-supplemented PN versus PN including all randomized controlled trials (RCTs) following the PRISMA statement [13].
We performed a systematic review of literature published between 1 January 1990 and 30 April 2013. We performed a comprehensive search of abstracts in the MEDLINE, OVID, Springer, and Cochrane Library database with the use of the following search terms: "Severe Acute Pancreatitis [Title]" or "Acute Necrotizing Pancreatitis [Title]", and following limited “Parenteral Nutrition [Title/ Abstract] with limitations to RCTs, Humans. Reports in English language were eligible for inclusion. Furthermore, additional studies were searched manually, showing reference lists of all retrieved articles, which were lost by the electronic search.
Only RCTs were adopted. Acute pancreatitis patients include those who diagnosed by Atlanta classification, and those with severe diseases assessed by APACHE II criteria and/or Ranson criteria, and/or Balthazar CT criteria. Any etiology was eligible, and there was no limitation of race, and sex distribution except age (<16 years old). Comparator intervention was considered pharmaconutrition-supplemented PN, while control intervention was considered PN. The included studies were required to report at least one of the following outcome measures: infection, mortality, intensive care unit (ICU) stay, hospital stay, and leukocytes change.
Reviewers investigated the risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions. Two data extractors compared the articles for participating institutions and inclusion criteria, avoiding double counting. The potential risk bias in the overall results resulting from the inclusion of studies were determined, and violated some of the eligibility criteria, sensitivity analysis and publication bias analysis were performed. Consensus was achieved through discussion when necessary.
Excel 2003 (Microsoft, Redmond, Wash) software was used for ours meta-analysis.
Two independent reviewers extracted data and entered it into the freeware program Review Manager (Version 5.1 for Windows, Cochrane Collaboration, Oxford, UK, 2008) respectively. The random-effects model was applied, and the odds ratio(OR) for each trial was calculated from the number of evaluable patients, and ORs with their twosided 95% CIs were used for dichotomous outcomes as test criterion and the confirmatory effect size estimate. Weighted mean difference (WMD) was calculated with 95% confidence intervals for continuous variables. Reported the same or similar outcomes were combined, which data extracted from different trials. P values were used for illustration, and the hypothesis tests were based on the 95% CIs.
A total of 1379 studies were retrieved, and the process of identifying relevant trials is shown in Figure 1. Among these 1379 studies, four RCTs [11, 12, 14, 15] were potentially appropriate clinical trials to be included in the metaanalysis which were pharmaconutrition-supplemented PN versus PN for SAP, which were all published as full articles. There were a total of 76 patients underwent pharmaconutrition-supplemented PN and 77 patients underwent PN for SAP. The principal characteristics of all the included studies are reported in Table 1.
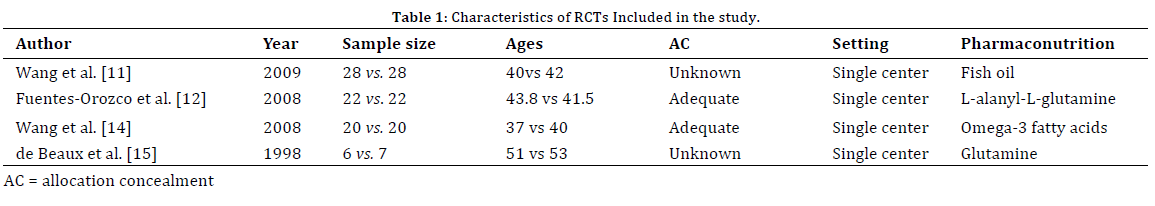
Two trials [11, 15] did not provide the allocation concealment, but other two trials [12, 14] elucidated the specific method of randomization (Table 1). Four studies provided administering compositions of nutritional support for PN and pharmaconutrition-supplemented PN. Pharmaconutrition-supplemented PN had two kinds in our included RCTs, which were glutamine [12, 15] and ω-3 fatty acids [11, 14] (Table 1). One RCT [12] mentioned the nutritional status through the nitrogen balance, given that lost-to-follow-up cases did not result in any censored data and that all of the outcomes in our study were admission. All four trials provided the most commonly analyzed outcomes for pharmaconutrition-supplemented PN. Moreover, because statistically significant data are more likely to be published, our meta-analysis was likely to be influenced very little by publication bias. However, because of four RCTs available, more detailed stratification comparisons could not make for our study, which would influence the validity of our study to some extent.
Infection included intraabdominal and extra-abdominal infections, such as infected pancreatic necrosis, pancreatic abscess, generalized peritoneal infection, pneumonia, bacteremia, urinary tract infection, and catheter-related sepsis.
3 included RCTs and involved 140 patients reported infection. The rate of infection in pharmaconutritionsupplemented PN group and PN group with SAP was 25.7% (18/70) and 42.9% (30/70) respectively. Metaanalysis showed significant difference in infection between pharmaconutrition-supplemented PN group and PN group. (OR, 0.42; 95% CI, 0.20–0.91; P =0.03) (Figure 2).
Four included RCTs and 153 involved patients reported the mortality. The mortality of pharmaconutritionsupplemented PN group and PN group was 2.6% (2/76) and 10.4% (8/77) respectively. Meta-analysis showed no significant difference in mortality pharmaconutritionsupplemented PN and PN for SAP (OR, 0.30; 95% CI, 0.07– 1.19; P=0.09) (Figure 3).
Two trials provided information regarding ICU stay which involved 84 patients. The random-effects model was used because heterogeneity was not detected. Pharmaconutrition-supplemented PN was not associated with a significantly shorter ICU stay than the control (MD, -3.65; 95% CI, -9.39–2.10; P =0.21) (Figure 4).
Two trials provided information regarding hospital stay which involved 84 patients. Because heterogeneity was not detected, the random-effects model was used. PN was not associated with a significantly longer hospital stay than pharmaconutrition -supplemented PN (MD, -1.20; 95% CI, -9.89–7.48; P =0.79) (Figure 5).
Two trials provided information regarding leukocytes change which involved 84 patients. The random-effects model was used which heterogeneity was not detected. At admission, pharmaconutrition-supplemented PN was associated with a significantly higher leukocytes change than the control (MD, 0.93; 95% CI, 0.21–1.65; P =0.01) (Figure 6a). After treated, pharmaconutritionsupplemented PN was associated with a significantly lower leukocytes change than PN (MD, -0.77; 95% CI, -1.47–-0.08; P =0.03) (Figure 6b). Compared with PN, pharmaconutrition-supplemented PN could decrease leukocytes change.
SAP is a critical illness which is accompanied with high morbidity and mortality. Despite advances in critical care during the past several decades, radiological, endoscopic, and surgical techniques, the management of SAP remains a challenge for most gastroenterologists. SAP, like trauma and sepsis, is characterized by a marked degree of protein breakdown, which catabolism of body protein may be as high as 2% per day [16-18]. In available managements, SAP posed a set of challenges with respect to nutritional support. Nutritional support can provide a long term nutritional support for SAP [19]. Early nutritional support is important to ensure optimum recovery, which start within 72 hours of onset of symptoms [20]. Feeding should therefore be initiated as soon as possible and be continued until adequate oral intake is achieved.
Several researches have emphasized the superiority of EN over PN for SAP [21, 22]. Suppression of pancreatic exocrine secretion by gastrointestinal rest used to be an important strategy to stabilize SAP, and PN was therefore advocated [23]. In patients with nausea, vomiting, and intermittent ileus secondary to intraabdominal infection or infected pancreatic and peripancreatic tissue, nutrient requirements cannot be met by EN, [24] and PN is still an option for these patients.
PN contributes to pancreas function and optimal recovery [25]. Many clinical studies pointed out that pharmaconutrition hastened the recovery of SAP patients, stimulated gastrointestinal motility, and alleviated the degree of SIRS [26, 27] Nutritional supplemented PN for SAP includes glutamine [12, 15] and ω-3 fatty acids [11, 14] which are pharmaconutrition. Glutamine, a conditionally essential amino acid, is an immunomodulatory agent, as it has beneficial effects on the cells of the immune system and their functions [28, 29] ω−3 fatty acids can alter cytokine production, enhance immunity, and reduce the rate of complications, for example, the incidence of organ dysfunction and the prevalence of nosocomial infections [30, 31] Previous studies have shown that glutamine-enriched PN formulas improve the prognosis of septic and surgical patients [22-25] by decreasing the acute inflammatory response commonly observed in such patients [3].
The present meta-analysis showed that pharmaconutritionsupplemented PN would be better results for infection (OR, 0.42; 95% CI, 0.20–0.91; P =0.03) and leukocytes change (before treated: mean different, 0.93; 95% CI, 0.21–1.65; P =0.01; after treated: mean different, -0.77; 95% CI, -1.47– -0.08; P =0.03). For pharmaconutrition-supplemented PN can reduce mortality, ICU stay, and hospital stay, our study showed it could do nothing for mortality (OR, 0.30; 95% CI, 0.07–1.19; P =0.09), ICU stay (mean different, -3.65; 95% CI, -9.39–2.10; P =0.21), and hospital stay (mean different, -1.20; 95% CI, -9.89–7.48; P =0.79).
The pathological mechanisms contributing to our outcomes are complex. Pharmaconutrition-supplemented PN offers a potential positive effect on the immune response. Many studies pointed out that pharmaconutrition have many effects on SAP: (1) stimulation of the production of antiinflammatory cytokines, (2) amelioration the course of infection by reduction of pro-inflammatory eicosanoids and cytokines, and (3) reduced bacterial translocation across the gut wall and thus reduced the risk of sepsis [11, 12, 14, 15, 32-34].
Acute pancreatitis is an acute inflammatory process of the pancreas, with variable involvement of other regional tissue and/or remote organ systems, such as SIRS and MODS [35]. Most complications and deaths that occur in SAP are because of inflammatory immune responses to pancreatic necrosis and/or infection [36]. Our result shows that attenuation of the hyperinflammatory response can be obtained by pharmaconutrition supplementation, which decreases infectious morbidity rate and declines leukocytes change in SAP. In generally, an anti-inflammatory reaction is initiated. It consists of a combination of excessive decrease of anti-inflammatory cytokines and impaired immune function which results in a reduction in mortality, duration of ICU stay, or duration of hospital stay. However, our study does not suggest these results.
Wang et al. reported that PN supplemented with omega-3 FAs diminished the hyperinflammatory response and the proinflammatory cytokine in severe acute pancreatitis [14], and found mega-3 FAs supplemented PN can elevate the IL-10 level and HLA-DR expression in SAP patients [11]. Fuentes-Orozco et al. suggested that treatment of patients with glutamine-supplemented PN decreased infectious morbidity rate compared with those who treated with nonenriched PN [12] De Beaux et al. reported that glutamine-supplemented PN improved lymphocyte proliferation and reduced proinflammatory cytokine release [15]. Although the meta-analysis are in line with research from Wang et al. [11, 14], Fuentes-Orozco et al. [12] and de Beaux et al. [15], our meta-analysis has some limitations. First, this meta-analysis included only 153 patients and 4 RCTs, and a type II error may be existence possibly, and our result may be real phenomena or may merely reflect the small sample number of those RCTs. Second, pharmaconutrition-supplemented PN is special, which may affect the clinic measures; Wang et al. used fish oil11 and omega-3 fatty acids [14], Fuentes-Orozco et al. used L-alanyl-L-glutamine [12], de Beaux et al. used glutamine [15]. Third, we cannot perform a subgroup analysis according to patient age and the etiology of disease, due to the lack of relevant information in the original works; thus it is unclear whether early pharmaconutrition is potentially feasible and effective in these subgroups of patients.
For patients with SAP, there are some problems for pharmaconutrition-supplemented PN. First, EN has been considered the method of choice for the nutrition of patients with SAP in recent years; Only pharmaconutritionsupplemented PN cannot maintain adequate treatment for the rapid progress, oral and enteral nutrition is considered as a therapeutic option for patients, and sometimes both forms of nutritional support may be administered in the same patient either contemporaneously or sequentially. Second, a long period of PN could increases the risk of an acute inflammatory response and septicemia [37, 38].
The current meta-analysis indicates that pharmaconutrition-supplemented PN only show advantages in infection and leukocytes change, as a new strategy had better treatment effect on SAP patients. Future large-scale, high-quality, multicenter trials are still required to clarify the issues of pharmaconutritionsupplemented PN for patients with SAP.
This work was supported by College Students Challenge Fund from League of Hubei University of Chinese Medicine, Scientific Research Fund from Hubei University of Chinese Medicine and Young Talents Project of Science and Technology Research Program from Hubei Provincial Department of Education (grant no. Q20132003).
Authors declare to have no conflict of interests.