Commentary - (2016) Volume 2, Issue 2
Gang Wang1, Wenhua Li2, Waqas Nawaz1, Xiaoqian Liao1, Meng-Qi Yang1, Li Zhang1, Farhan-Ullah Khan1, Xiaoming Qi3, Dingding Chen1, Zhijie Wang4, Feng Yu1, Lei Han5,6 and Xiaohui Zhou1,7*
1Department of Clinical Pharmacy, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu Province, China
2Department of Urology, Aviation General Hospital, Beijing, China
3Shanxi University of Traditional Chinese medicine, Taiyuan, Shanxi Province, China
4Key Laboratory of Semiconductor Materials Science, Institute of Semiconductors, Chinese Academy of Sciences, Beijing, China
5Department of Pharmacy, Jiangsu Worker Medical University, Nanjing, Jiangsu Province, China
6Department of Pharmacy, Jiangsu Jiankang Vocational College, Nanjing, Jiangsu Worker Medical University, Nanjing, Jiangsu Province, China
7Department of Surgery, Nanjing Shuiximen Hospital, Nanjing, Jiangsu Province, China
*Corresponding Author:
Xiaohui Zhou
Department of Clinical Pharmacy
School of Basic Medicine and Clinical Pharmacy
China Pharmaceutical University, Nanjing
Jiangsu Province, 211198, China
Tel: 008613515121656
E-mail: zhxh@cpu.edu.cn
Received date: November 30, 2016; Accepted date: December 23, 2016; Published date: December 26, 2016
Citation: Wang G, Li W, Nawaz W, et al. Efficacy of a Novel Epicardium Drug Delivery System for Bone Marrow Stem Cells Treating Heart Failure After Myocardial Infarction. Insights Stem Cells. 2016, 2:2.
Objective: In this study, we will demonstrate that the epicardium drug delivery system for bone marrow stem cells (EDDS & BMSCs) can treat heart failure.
Methods: 24 rats were randomly allocated into four groups and six animals in each group as follows: Normal group, heart failure after myocardial infarction (HF after MI) group, EDDS and EDDS & BMSCs group. EDDS were implanted in SD rat with post-infarction dilated heart failure. The bone marrow stem cells, which were delivered by the device of subcutaneous tube at post operation seventh days, fifteenth days and twenty-fifth days. Electrocardiogram was measured and blood samples were collected at post operation seventh days, fifteenth days and twentyfifth days. Measurements of rat cardiovascular hemodynamics were obtained at 30th day. The concentrations of BNP were quantified by ELSIA. Myocardial fibrosis was determined by Masson’s trichrome staining quantitative assay.
Results: EDDS and EDDS & BMSCs treatment increased ±dp/dt levels and decreased LVEDP level 30 days after MI. In HF after MI group the level of LVSP was 94.7 mmHg and increased to 109.5 mmHg (p<0.01) in EDDS & BMSCs group. Compared with the HF after MI group (70.5 μg/L), the level of BNP was significantly decreased in the EDDS & BMSCs group (48.0 μg/L, p<0.01) and EDDS group (51.9 μg/L, p<0.01). The area of fibrosis heart tissue was decreased in the EDDS & BMSCs group. The ECG was improved in the EDDS & BMSCs group.
Conclusions: Using BMSCs delivery by EDDS treated heart failure has treatment effect.
Keywords
Heart failure after myocardial infarction; Bone marrow stem cells; Myocardial infarction; Epicardium drug delivery system
List of Abbreviations:
SD: Sprague Dawley; BMSCs: Bone Marrow Stem Cells; EDDS: Epicardium Drug Delivery System; EDDS & BMSCs: Epicardium Drug Delivery System for Bone Marrow Stem Cells; HF after MI: Heart Failure after Myocardial Infarction; BNP: Brain Natriuretic Peptide; ELSIA: Enzyme-Linked Immunosorbent Assay; LVSP: Left Ventricle Systolic Pressure; HF: Heart Failure; CSD: Cardiac Support Device; QVR: Quantitative Ventricular Restraint; ECG: Electrocardiogram; LVEDP: Left Ventricle End-Diastolic Pressure; DMEM: Dulbecco Modified Eagle Medium; ANOVA: Analysis of Variance; LAD: Left Anterior Descending
Introduction
Heart failure (HF) is a complex clinical syndrome that can result from any functional or structural cardiac disorder that impairs the ventricle’s ability to fill with or eject blood [1,2]. Heart failure not only reduces the patient's life expectancy, but also causes the patient to breathe difficult, fluid retention and fatigue, which significantly reduce the quality of life of patients [3]. Almost all of the cardiovascular disease will eventually lead to heart failure, and myocardial infarction is the main cause of heart failure [4]. Heart failure after myocardial infarction can lead to left ventricular systolic and diastolic dysfunction [5]. In the end stage of heart failure, the effect of drugs treatment is very low [6]. Therefore, many studies have turned to use devices to therapy heart failure [7]. Ventricular restraint device was developed to treat severe heart failure, including CSD (CorCap; Acorn Cardiovascular Inc, St Paul, Minn), Heart net (Paracor Medical, Sunnyvale, Calif) and QVR (Polyzen) [8,9]. Ventricular restraint therapy can improve the adverse remodeling of the myocardium was shown in studies [10-12]. However, CSD and Heart Net take effect by passively physical shaping, and thus has great passivity in application [13]. Although quantitative ventricular restraint (QVR) was developed to measure and adjust the restraint level [14]. However, it cannot perform active and positive clinical intervention, especially direct medicine intervention, and cannot be combined with modern medicine treatment of various species and good effect. Therefore, those devices clinical treating effect and treating meaning are discounted and need to be improved.
At the same time, recent studies have found that bone marrow mesenchymal stem cells (BMSCs) have myocardial plasticity that can complement the death of cardiac muscle cells, promote angiogenesis, thereby can repair damaged myocardium and improve cardiac function [15-17]. Autologous BMSCs can be obtained by bone marrow puncture, and there is no immune rejection in autologous transfusion [18]. At present, there are have two ways use stem cells to treat heart failure, one is systemic administration and the other is a local disposable. However, these two kinds of ways have drawbacks, the systemic administration have significant side effects and local disposable is lack of flexibility [19].
Therefore, we recently developed an epicardium drug delivery system (EDDS) on the surface of the heart. The device includes a net cover for surrounding a ventricle and is formed by hollow tubes. All the hollow tubes can completely communicate with each other. The hollow tubular structure can be filled with various kinds of liquid of different drugs or cells characteristics, and then the corresponding reaction pressure generated can be applied to the ventricle and the surfaces of the heart. The device also can be incorporated with local administration. Accordingly, the medicine compound or cells in the fluid inside the tubes can affect on cardiac muscle directly, and produce direct pharmacological action under high local concentration on cardiac muscle or other heart tissues without entering blood circulation [19]. The net cover is connected to two implantable medical grade tubes to easy delivery. The net cover combine with medicine, then will produce medical treatment effect, so as to combines with the heart wall support effect [8].
We hypothesize that BMSCs delivery by epicardium drug delivery system three times treated heart failure after myocardial infarction to investigate a novel treatment method for heart failure.
Materials and Methods
Experimental animals
Healthy male SD rats weighing from 240 g to 260 g were obtained from experimental animal center of Zhejiang Province. The animals were maintained under standard laboratory conditions. This study was approved by the animal ethical committee of the China Pharmaceutical University. All rats were randomly divided into four groups and six animals in each group. Normal group, HF after MI group, epicardium drug delivery system (EDDS) group and epicardium drug delivery system for BMSCs (EDDS & BMSCs) Group. The left coronary artery ligation was performed in 18 rats. A MI is defined as the ECG shows ST elevation or depression. Rats of MI were randomly allocated into four groups as follows: HF after MI group: do not take any intervention measures after MI; EDDS group: implantation EDDS treated after MI; EDDS & BMSCs group: The bone marrow stem cells, which were delivered by the system of subcutaneous tube at post operation seventh days, fifteenth days and twenty-fifth days. All animals in the operation were measured ECG at postoperative 25 min. Blood samples were collected and electrocardiogram was measured in all rats at post operation 7th day, 15th day and 30th day. In this study, we use BL420-type physiological signal acquisition system (Chengdu Instrument Company) to record the left ventricle systolic pressure (LVSP), the left ventricle end-diastolic pressure (LVEDP) and the maximum increases and decreases in the left ventricle pressures (±dP/dtmax) at post operation 30th day. The heart tissue was immersed in a 10% neutral buffered formalin. Sections were taken from heart tissues of each rat. A double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) was used to detect the serum BNP.
Anesthesia and postoperative care
Anesthesia was maintained with 10% chloral hydrate (0.3 ml/100 mg). Postoperatively, animals received penicillin sodium (5 wU/100 g intramuscularly every 24 h for 5 days) for antibiotic prophylaxis. All rats were carried out in strict accordance with the recommendations in the Guide of China Pharmaceutical University for the care.
Bone marrow mesenchymal stem cell (BMSC) isolation
Rats were anesthetized by an intraperitoneal injection of 10% hydral. The femurs and tibias were removed, and the two terminal regions of these bones were cut and flushed with DMEM to collect cells. The cell mixture was centrifuged at 1500 rp/min for 10 min, to prepare a single-cell suspension, and the supernatant was discarded. The BMSCs were isolated with Percoll and resuspended in DMEM [20]. So, we looked at BMSCs in Figure 1.
Figure 1: Image of isolated BMSCs (4x10).
Figure 2A-2E provides images of an EDDS. EDDS was implanted via 3, 4 intercostal incisions and pericardiotomy. The EDDS was placed over the heart to completely envelop both ventricles and secured to the atrioventricular groove using interrupted sutures (Figure 2F and 2G). The drug tubes were then tunneled through the second interspace and secured to the back. The incisions were then closed in layers and the animal recovered.
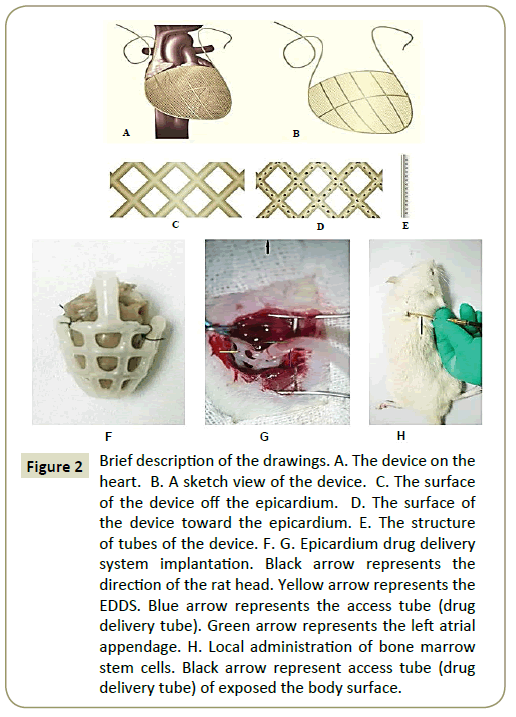
Figure 2: Brief description of the drawings. A. The device on the heart. B. A sketch view of the device. C. The surface of the device off the epicardium. D. The surface of the device toward the epicardium. E. The structure of tubes of the device. F. G. Epicardium drug delivery system implantation. Black arrow represents the direction of the rat head. Yellow arrow represents the EDDS. Blue arrow represents the access tube (drug delivery tube). Green arrow represents the left atrial appendage. H. Local administration of bone marrow stem cells. Black arrow represent access tube (drug delivery tube) of exposed the body surface.
BMSCs is filled inside the system using 1 ml syringe absorbed with 0.5 ml (2*10^6/ml) (Figure 2H).
ECG recording
Following the induction of anesthesia, the rats were fixed on the operating table. A BL420-type physiological signal acquisition system (Chengdu Instrument Company) was used to measure ECG.
Detection of the hemodynamic response
The rats were fixed on the operating table after the induction of anesthesia. A longitudinal incision was cut in the neck skin and the right common carotid artery was isolated. The Distal common carotid artery was ligated and the proximal was clipped with an artery clamp (Figure 3). BL420-type physiological signal acquisition system (Chengdu Instrument Company) was used to record the left ventricle systolic pressure (LVSP), the left ventricle end-diastolic pressure (LVEDP) and the maximum increases and decreases in the left ventricle pressures (±dP/dtmax) at postoperation 30th day.
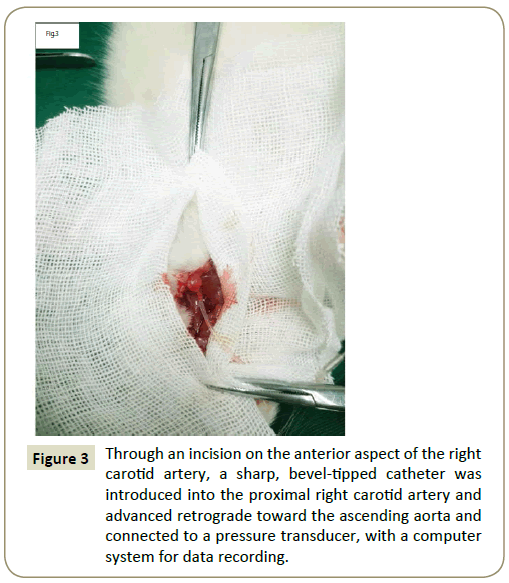
Figure 3: Through an incision on the anterior aspect of the right carotid artery, a sharp, bevel-tipped catheter was introduced into the proximal right carotid artery and advanced retrograde toward the ascending aorta and connected to a pressure transducer, with a computer system for data recording.
Myocardial histological examination
The heart tissue is submerged in 10% neutral buffered formalin for 48 h. Sections were taken from heart tissues of each rat, dehydrated in gradual ethanol, cleared in toluene and infiltrated in paraffin using an automatic tissue processor (Jinhua, Zhejiang Cody Equipment Co., Ltd.). They were then embedded in molten paraffin. Thick sections were cut at 5 μm with a rotary microtome, processed in toluene-ethanol series and stained with Masson dye. They were finally observed under photonic microscope for histopathological changes.
Determination of plasma BNP content
We centrifuged 1 ml of venous blood at 3000 rpm for 15 min and the plasma was separated. ELISA kit (Shanghai, Jinma biological Ltd.) was used for BNP detection according to the manufacturer’s instructions.
Statistical analysis
The replicate is biological.
All values were expressed as mean ± S.D. of six replicates and analyzed with one way analysis of variance (ANOVA) using SPSS (19.0) statistical software. Differences between groups and times were analyzed by ANOVA. Two-group comparison was performed by LSD-q test. P value of less than 0.05 was considered statistically significant.
Results
ECG
The ECG of various time periods was shown in Figure 4 (a1-5, b1, c1, d1,) ECG shows normal ST-T wave, no significant Q wave, isoelectric ST segment and upright T wave in the normal group and pre-operation of all rats. (b2, c2, d2,) ECG shows marked ST elevation with hyperacute T wave changes at 25 min after ligation. At 7th days after ligation, (b3) ECG shows ST segment elevation in the HF after MI group. (c3) ECG shows T waves inversion in the EDDS group. (d3) ECG shows T waves inversion in the EDDS & BMSCs group. At 15th days after ligation, (b4) ECG shows pathologic Q waves with ST segment elevation in the HF after MI group. (c4) ECG shows T waves inversion in the EDDS group. (d4) ECG shows ST segment normal in the EDDS & BMSCs group. At 30th after ligation, (b5) ECG shows pathologic Q waves with ST segment elevation in the HF after MI group. (c5) ECG shows pathologic Q waves with T waves inversion in the EDDS group. (d5) ECG shows normal ST-T waves and no significant Q wave in the EDDS & BMSCs group.
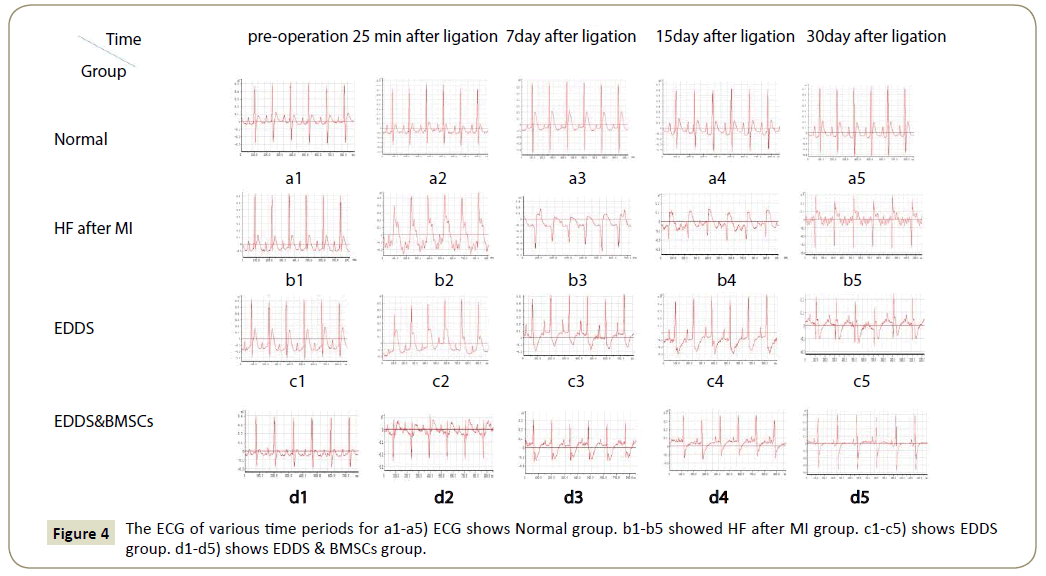
Figure 4: The ECG of various time periods for a1-a5) ECG shows Normal group. b1-b5 showed HF after MI group. c1-c5) shows EDDS group. d1-d5) shows EDDS & BMSCs group.
Morphological examination of the myocardial tissue
We assessed the size of the fibrotic area using Masson trichrome staining (Figure 5). 30 days after LAD ligation characteristic signs of late phase post infarction remodeling of the heart were visible. The blue region of HF after MI group and EDDS group were myocardial fibrosis; normal group and EDDS & BMSCs group were normal in red.
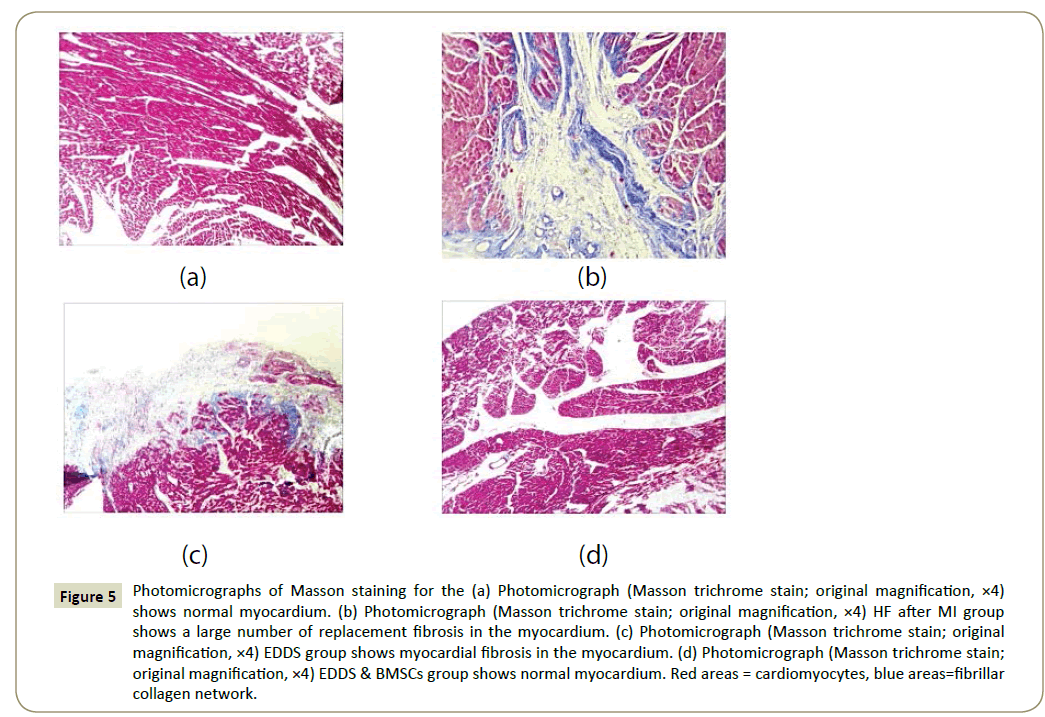
Figure 5: Photomicrographs of Masson staining for the (a) Photomicrograph (Masson trichrome stain; original magnification, ×4) shows normal myocardium. (b) Photomicrograph (Masson trichrome stain; original magnification, ×4) HF after MI group shows a large number of replacement fibrosis in the myocardium. (c) Photomicrograph (Masson trichrome stain; original magnification, ×4) EDDS group shows myocardial fibrosis in the myocardium. (d) Photomicrograph (Masson trichrome stain; original magnification, ×4) EDDS & BMSCs group shows normal myocardium. Red areas = cardiomyocytes, blue areas=fibrillar collagen network.
Hemodynamic results
Changes in the hemodynamic parameters at 30 days after LAD ligation. As shown in Figure 6, in normal group the level of LVSP was 116.5 mmHg and declined to 94.7 mmHg (p<0.01) in HF after MI group. In normal group the level of LVEDP was 1.8 mmHg and increased to 14.7 mmHg (p<0.01) in HF after MI group, and the +dP/dtmax and -dP/dtmax levels were significantly decreased in the HF after MI. Detailed examination of hemodynamic parameters confirmed a severely decreased cardiac function in all HF after MI group. EDDS and EDDS & BMSCs treatment increased ±dp/dt levels and decreased LVEDP level 30 days after MI. In HF after MI group the level of LVSP was 94.7 mmHg and increased to 109.5 mmHg (p<0.01) in EDDS & BMSCs group, whereas EDDS group did not significantly increased the level of LVSP.
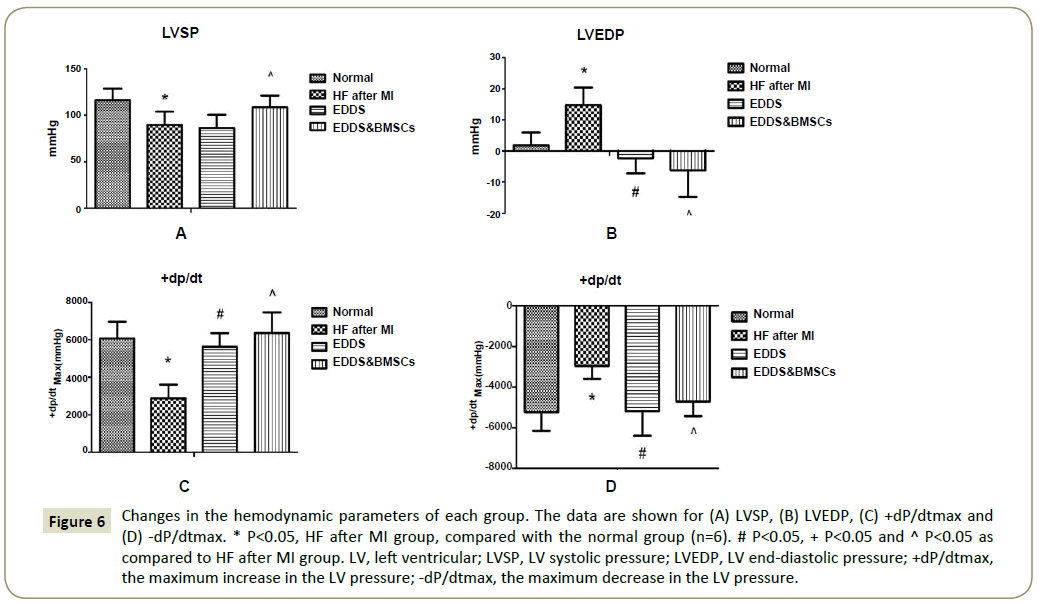
Figure 6: Changes in the hemodynamic parameters of each group. The data are shown for (A) LVSP, (B) LVEDP, (C) +dP/dtmax and (D) -dP/dtmax. * P<0.05, HF after MI group, compared with the normal group (n=6). # P<0.05, + P<0.05 and ^ P<0.05 as compared to HF after MI group. LV, left ventricular; LVSP, LV systolic pressure; LVEDP, LV end-diastolic pressure; +dP/dtmax, the maximum increase in the LV pressure; -dP/dtmax, the maximum decrease in the LV pressure.
The level of plasma BNP
Plasma BNP level in different age groups was shown in Figure 7 Compared with the normal group (55.2 μg/L), the level of BNP was significantly increased in the HF after MI group (70.5 μg/L, p<0.05). But, compared with the HF after MI group (70.5 μg/L), the level of BNP was significantly decreased in the EDDS & BMSCs group (48.0 μg/L, p<0.05) and EDDS group (51.9 μg/L, p<0.05).
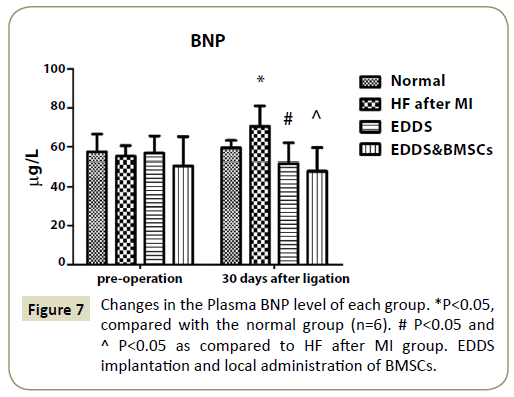
Figure 7: Changes in the Plasma BNP level of each group. *P<0.05, compared with the normal group (n=6). # P<0.05 and ^ P<0.05 as compared to HF after MI group. EDDS implantation and local administration of BMSCs.
Discussions
CSD and Heart Net takes effect by passively physical shaping, but cannot combine with drug to treat heart failure. In addition, due to the properties of the material itself, leading to restrain too large or too small and once the implant is unable to change the effect of treatment [21,22]. We recently introduced EDDS as a platform to restraint ventricle and deliver BMSCs to treat heart failure [23]. Using this system, we showed that BMSCs delivery have a great effect and reverse remodeling. Such studies have not been reported.
In this research, we implant EDDS to deliver BMSCs into epicardium through the access tube. The hollow tubular structure can be filled with BMSCs, and then the corresponding reaction pressure generated can be applied to the ventricle and the surfaces of the heart. The bone marrow stem cells to replace or repair damaged heart muscle.
The drug of BMSCs, which were delivered by the system of subcutaneous tube at post operation seventh days, fifteenth days and twenty-fifth days. ST segment elevation or Pathologic Q waves with T wave inversion was indicated necrosis and fibrosis after ligation of the left coronary artery [24,25]. Electrocardiogram was measured and blood samples were collected at post operation 7th days, 15th days and 30th days. HF after MI group appeared ST segment elevation and pathological Q waves, which showed myocardial infarction. Pathologic Q waves with T wave inversion and ST segment elevation were indicated myocardial infarction in the EDDS group. ST segment depression was shown in the EDDS & BMSCs group at post-operation 7th days. The ECG return to normal at post-operation 15th days and 30th days after ligation. Prompt BMSCs delivery by EDDS to treat heart failure has a therapeutic effect.
Compared with the normal group, the levels of BNP and LVEDP were significantly increased in the HF after MI group. The +dP/dtmax, -dP/dtmax and LVSP levels were significantly decreased. The area of MI was significantly increased. Those results confirmed that the rats of HF after MI group exhibited clear myocardial fibrosis, reduced heart function and ventricular remodeling [26,27]. Compared with the HF after MI group, the levels of BNP and LVEDP were significantly decreased in the EDDS group. The +dP/dtmax and -dP/dtmax levels were significantly increased, while the areas of MI were not significantly difference. Those data revealed that the rats of implantation EDDS can restore heart function. But the degree of fibrosis was not significantly decreased. The levels of BNP and LVEDP were significantly decreased in the EDDS & BMSCs group. The LVEDP levels increased was the main mark of heart failure. Thus, there are some research have shown that the heart function could be improved via the device of surrounding the ventricular wall [28]. The EDDS support the ventricular wall during the ventricular diastolic. The negative values appeared from EDDS and EDDS & BMSC groups in the data of LVEDP have shown that the supporting forces of EDDS was effective for ventricular wall. Our model assumes that the slack length of the silicon is at early diastolic filling and includes fabric pre-stretch of 5%. However, the optimal amount of pre-stretch is unknown. The ±dp/dt levels were significantly increased. The degree of fibrosis was significantly decreased. Those results revealed that EDDS & BMSCs group could restore heart function and improve it, decreased fibrosis and attenuated ventricular remodeling. This research has demonstrated that BMSCs delivery by EDDS was able to inhibit or reverse remodeling [27]. The method using this system deliver BMSCs maybe can inhibit or even reverse the ventricular remodeling, which is superior to the single ventricular restraint device for the treatment of heart failure [9,29].
The Limitations of the experiment was not set different size of EDDS. So, we selected the rats (250 g + 10 g) as object of study, which the EDDS can match the heart of most of the experimental animals. In the follow-up study, we will try to design varieties of size of the EDDS to solve this problem.
The study demonstrated that the use of EDDS combine with bone marrow stem cells in the treatment of heart failure is feasible and have a good effect. The system can flexible to apply stem cell to therapy heart failure is the highlight of this study. EDDS combined with other drugs or stem cells to therapy heart failure, which achieve a better effect in our future studies [30-32].
Ethics Statement
All applicable international, national, and institutional guidelines for the care and use of animals were followed. The protocol was approved by the Committee on the Ethics of Animal Experiments of the China Pharmaceutical University. All surgery was performed under chloral hydrate anesthesia, and all efforts were made to minimize suffering.
Acknowledgment
The author thanks to the participants who take part in this research. We are grateful to Michael Deininger (Medical innovation center university of Michigan), and Zhijie Wang (Chinese academy of science, Beijing) for their cooperation, encouraging and supporting role in our research. We also thank Xue Li from North University of China for his help in accomplishing the 3D graphics of EDDS.
Author Contributions
Zhou Xiaohui designed experiments; Wang Gang carried out experiments; Waqas Nawaz analyzed experimental results. Li Wenhua analyzed sequencing data and developed analysis tools. Liao Xiaoqian assisted with Illumina sequencing. Han Lei wrote the manuscript.