Keywords
Transthyretin; Oleuropein aglycone; Amyloid; FAP; FAC
Abbreviations
TTR: Transthyretin; SSA: Senile Systemic Amyloidosis; FAP: Familial Amyloid Polyneuropathy; FAC: Familial Amyloid Cardiomyopathy; AD: Alzheimer’s Disease; APP: Amyloid Precursor Protein; hIAPP: Amylin or Human Islet Amyloid Polypeptide; wt-TTR: Wild-Type TTR; OleA: Oleuropein Aglycone; NDGA: Nordihydroguaiaretic Acid; EGCG: Epigallocatechin 3-Gallate; GM1: Monosialotetrahexosylganglioside 1
Oleuropein Aglycone and Protein Aggregation
Diet in human health is no longer simple nutrition, but in light of recent epidemiological studies, it is may have deep effects on modulating apoptosis, detoxification, appropriate gene response and protection against disease. Recently, nutrigenomics research suggested that it is not just deficiency of a particular element but also the presence in diet in adequate amount of various other compounds from fruits and vegetables that play a role in decreasing risk of a range of disease including cancer cardiovascular and neurodegenerative disease. Our laboratory focused attention on the main phenolic compound, Oleuropein aglycone (OleA), of extra-virgin olive oil. The pharmacological properties of olive oil, especially the olive fruit and its leaves have been recognized as important components of medicine and a healthy diet for their phenolic content [1]. OleA has several pharmacological properties such as antioxidant [1], anti-inflammatory [2] anti-atherogenic [3], anti-cancer [4], antimicrobial [5] and antiviral [6], and for these reasons, it is commercially available as food supplement. In addition, it has been shown to be cardioprotective against acute adriamycin cardiotoxicity [7] and has been shown to exhibit antiischemic and hypolipidemic activities [8]. Another important aspect to be considered is that the OleA interaction with cell membranes would increase its local concentration. This aspect is particularly relevant when considering the beneficial effects that would be attained above critical concentration of the molecule.
Recently, most of the research on OleA was focused on its anti-amyloidogenic activity, instead it resulted be able: to interfere in vitro with amyloid fibril formation of two proteins, Amylin, or human islet amyloid polypeptide (hIAPP), and Aβ42, associated with type 2 diabetes and Alzheimer’s disease (AD), respectively [9,10]; studies in vivo, using C. elegans as a simplified invertebrate model of AD [11], showed that this polyphenol skips the appearance of toxic oligomers promoting peptide aggregation into aggregates devoid of cytotoxicity [12]. Finally, we recently showed that a dietary supplementation of OleA strongly improved the cognitive performance of the TgCRND8 mouse model of AD; mice showed remarkably reduced plaque deposits, microglia migration to the plaques for phagocytosis [13]. Data obtained with cultured cells confirmed that OleA is also able to induce autophagy, possibly by acting on the mTOR pathway [13]. OleA was more effective than Oleuropein and hydroxytyrosol (they are also effective but to a lesser extent) as inhibitor of Tau fibrillization [14]. OleA was also shown to modify Amyloid Precursor Protein, (APP) processing, increasing the formation of the non-amyloidogenic and neuroprotective sAPPα fragment and to decrease Aβ oligomers in HEK695 cell supernatants by increasing matrix metalloproteinase-9 (MMP- 9) secretion [15]. These results are very promising and pave the way to a more extensive investigation on OleA ability to inhibit toxic amyloid aggregation of many other amyloid-associated peptides/proteins. Indeed, the consensus idea that the protein misfolding is the cause of several different diseases and the high similarity of amyloid aggregates originated from different protein/peptides, gives the opportunity to develop common therapies for these devastating diseases. The present review will describe the biological effects of OleA with particular attention on the molecular mechanism underlying the protective action against TTR amyloidosis toxicity.
Transthyretin Related Amyloidosis
TTR is one of the 25 known proteins that are capable of forming amyloid fibrils in vivo. TTR amyloidosis is a systemic disorder characterized by the extracellular deposition of amyloid fibrils composed of TTR. TTR amyloidosis include senile systemic amyloidosis (SSA), familial amyloid cardiomyopathy (FAC), familial amyloid polyneuropathy (FAP), and central nervous system selective amyloidosis (CNSA). Over 80 disease-causing mutations in the TTR gene are known in FAP, most of which reduce tetramer stability increasing the fraction of aggregation-prone unstable dimeric and monomeric protein. However, although specific mutations are required for FAP development, different genetic factors may govern the age of onset and the occurrence of disease anticipation [16]. Instead, fibrils in FAP patients are composed of single-site mutant TTR and among the numerous pathogenic variants Leu55→Pro55 (L55P) is highly amyloidogenic and forms amyloid fibrils in vitro. Treatment of familial TTR amyloidosis has historically relied on liver transplantation as a crude form of gene therapy [17]. As TTR is primarily produced in the liver, replacement of the liver gene carrying the mutation with the normal form is able to reduce the mutant TTR levels in the body to < 5% of pre-transplant levels. Some mutations, however, cause CNS amyloidosis, and due to their production by the choroid plexus, these forms do not respond to liver transplantation.
Without adequate therapy, TTR amyloidosis results in a fatal outcome and lead to death within 10-15 years mainly following heart complications. Indeed, the presence of TTR aggregates in the heart parenchyma, may represent a basis for the occurrence of rhythm disturbances and conduction alterations often seen in patients with TTR amyloidosis. However, besides liver and heart transplantation, there is no effective medical treatment to enhance or to block disease progression.
On this point, it also appears of growing importance to search for compounds able to interfere with protein aggregation either by stabilizing native states against unfolding and by skipping the growth of toxic aggregation intermediates providing tools to counteract, or to slow down, the appearance and progression of diseases.
Transthyretin and Polyphenols
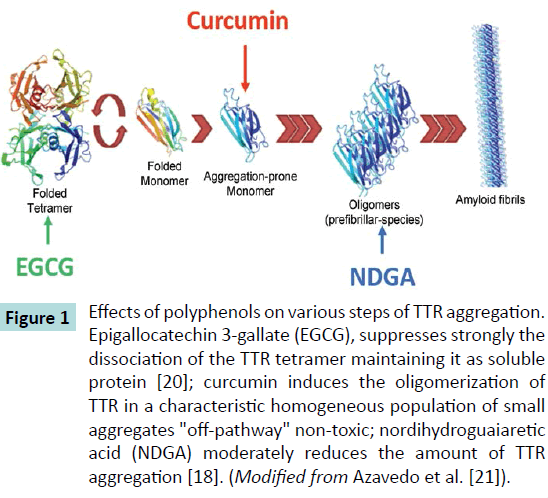
Several natural polyphenols have been reported to act on different amyloidogenic proteins inhibiting amyloid formation (see the following section). In particular, for TTR, the attention is focused on some natural polyphenols (Figure 1) such as curcumin, nordihydroguaiaretic acid (NDGA) and epigallocatechin 3-gallate (EGCG) that have structure similarities with T4. These studies showed that all these compounds stabilize the TTR and inhibit amyloid aggregation by three different means: EGCG strongly suppresses the dissociation of the TTR tetramer maintaining it as a soluble protein; curcumin induces the oligomerization of TTR in a characteristic homogeneous population of non-toxic "offpathway" small aggregates and NDGA moderately reduces the amount of aggregated TTR [18]. Furthermore, the oligomers and the intermediate species formed with the treatment of EGCG and curcumin are not toxic to the neuronal cells [19].
Figure 1 Effects of polyphenols on various steps of TTR aggregation.
Epigallocatechin 3-gallate (EGCG), suppresses strongly the
dissociation of the TTR tetramer maintaining it as soluble
protein [20]; curcumin induces the oligomerization of
TTR in a characteristic homogeneous population of small
aggregates "off-pathway" non-toxic; nordihydroguaiaretic
acid (NDGA) moderately reduces the amount of TTR
aggregation [18]. (Modified from Azavedo et al. [21]).
Oleuropein Aglycone Reduces Transthyretin Fibrils Formation
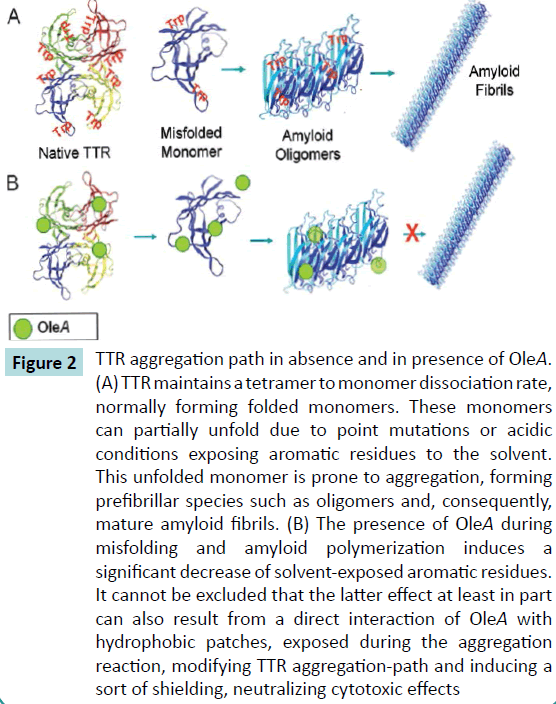
The knowledge of the mechanism by which polyphenolic compounds block amyloid formation is of considerable importance, as amyloid aggregation is a common feature of many degenerative diseases including Alzheimer’s, type II diabetes, Parkinson’s, Creutzfield-Jacob’s, Huntington’s and many others. The selection and development of small molecules able to inhibit the formation of toxic protein assemblies or to reduce their ability to bind to cell membrane could have a considerable clinical application, making a determination of the mechanism by which polyphenols disrupt aggregated conformations essential for structure-activity assays. Recent papers suggested that aromatic interactions favour molecular recognition of amyloidogenic sequences by enhancing the directionality and orientation needed for the ordered self-assembly process and hence fibril assembly kinetics [22]. On the other hand, it has been reported that several polyphenolic compounds are able to interact with amyloidogenic aromatic residues hindering π-system stacking [23,24] and inhibiting the elongation phase of fibril growth or the assembly of large oligomers without interfering with early nucleation events [25]. Our biophysical analysis [26] showed that OleA is not able to inhibit TTR aggregation of both wt- and L55PTTR but it reduces the exposition of protein aromatic residues during the amyloidogenic process. We found that the presence of OleA during wt-TTR and L55P-TTR aggregation pathway induced an increase of Trp quenching, suggesting that the effect of OleA in stabilizing meta-stable intermediate states relies on the reduction of the overall surface hydrophobicity of the aggregates (Figure 2). OleA does not prevent TTR aggregation by itself but, rather, it is effective in counteracting mature fibrils growth, a behaviour comparable with previously data shown with tau [27], amylin [9] and Abeta peptides [10]. The reported data suggest that OleA induces some remodelling of the supramolecular structure of the growing aggregates. This implies a mechanism of action different from that merely ascribable to some stabilization of the protein native structure hindering misfolding and aggregation, as observed for other anti-amyloidogenic compounds [28,29].
Figure 2 TTR aggregation path in absence and in presence of OleA.
(A) TTR maintains a tetramer to monomer dissociation rate,
normally forming folded monomers. These monomers
can partially unfold due to point mutations or acidic
conditions exposing aromatic residues to the solvent.
This unfolded monomer is prone to aggregation, forming
prefibrillar species such as oligomers and, consequently,
mature amyloid fibrils. (B) The presence of OleA during
misfolding and amyloid polymerization induces a
significant decrease of solvent-exposed aromatic residues.
It cannot be excluded that the latter effect at least in part
can also result from a direct interaction of OleA with
hydrophobic patches, exposed during the aggregation
reaction, modifying TTR aggregation-path and inducing a
sort of shielding, neutralizing cytotoxic effects
Oleuropein Aglycone is Cytoprotective against TTR Toxicity
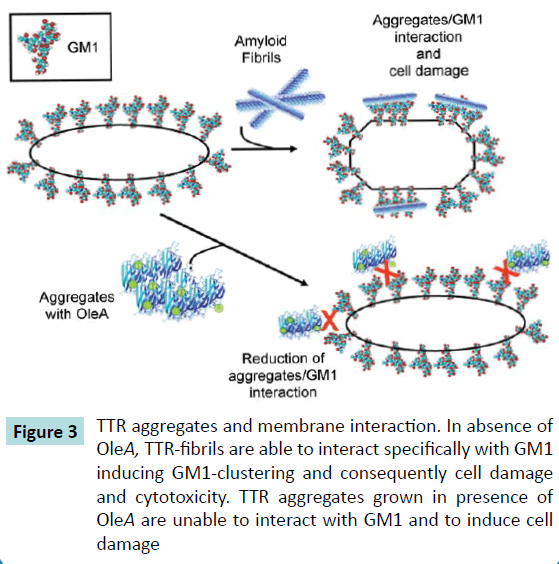
Considering that amyloid aggregates can bind to a large number of biological molecules that range from glycosaminoglycans (e.g. GM1) and nucleic acids to a variety of proteins and lipids, the change in membrane dynamics observed for GM1, following the binding of amyloid aggregates, may imply that amyloids can potentially harm all those cellular mechanisms that base their efficiency on molecules mobility [30]. Actually, growing evidence shows that amyloid aggregates can alter membrane mobility of a number of proteins and other plasma membrane components, leading either to a gain or to a loss of function. For example, the binding of Sup35 amyloid fibrils to the plasma membrane can cause an accumulation of Fas receptors associated with GM1 with subsequent activation of the extrinsic apoptotic pathway [31]. On the other hand, sequestration of neurotransmitter receptors, such as the metabotropic glutamate mGluR5, by Aβ1- 42 oligomers has been shown to impair intracellular calcium levels and synaptic network activity [32]. In this context, an alteration of GM1 mobility may compromise its regulatory role in neurodevelopment and neuroprotection [33,34], or influence cellular pathways linked to raft dynamics.
It is widely reported that a main mechanism of amyloid aggregate cytotoxicity requires the primary interaction with the cell membrane, which results in functional and/or structural perturbation of the latter. Emerging evidence points to fibrils/ membrane interaction as a key factor of the cytotoxic potential of amyloid aggregates [35,36]. Indeed, alterations of membrane fluidity resulting from TTR-aggregates interaction with neuronal cells were previously reported to be a key event in protein cytotoxicity [37]; this evidence results also from our study where both wt- and L55P-TTR fibrils appeared able to interact specifically with membrane GM1 of cardiomyocyte cells line [26]. Our study indicates that OleA protection results from a different mechanism whereby its interaction with TTR does not stabilize the tetramer structure or hinder TTR transition to an amyloid structure, rather, OleA interferes with TTR fibrillation by stabilizing oligomer-like intermediates and changing the surface properties of TTR/OleA aggregates. Accordingly, the presence of OleA during TTR aggregation induces a significant reduction of TTR aggregates-GM1 interaction, with consequently loss of cytotoxicity (Figure 3).
Figure 3 TTR aggregates and membrane interaction. In absence of
OleA, TTR-fibrils are able to interact specifically with GM1
inducing GM1-clustering and consequently cell damage
and cytotoxicity. TTR aggregates grown in presence of
OleA are unable to interact with GM1 and to induce cell
damage
These data suggest that OleA, or its molecular scaffold can be a good starting point to design novel therapeutic strategies for prevention and therapy of TTR-associated sporadic or familial amyloidosis.
Acknowledgment
This project was supported by Italian MIUR, PRIN 2009 (2009KN2FBM_002) and by Fondazione Cassa di Risparmio Pistoia e Pescia (Project n
References
- Visioli F, Poli A, Galli C (2002) Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev 22: 65-75.
- Visioli F, Bellosta S, Galli C (1998) Oleuropein, the bitter principles of olives, enhances nitric oxide production by mouse macrophages. Life Sci 62: 541-546.
- Carluccio MA, Siculella L, Ancora MA, Massaro M, Scoditti E, et al. (2003) Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of mediterranean diet phytochemicals. Arterioscler Thromb Vasc Biol 23: 622-629.
- Owen RW, Giacosa, A, Hull WE, Haubner R, Spiegelhalder B, et al. (2000) The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur J Cancer 36: 1235-1247.
- Tripoli E, Giammanco M, Tabacchi G, Di Majo D, Giammanco S, et al. (2005) The phenolic composition of olive oil: structure, biological activity, and beneficial effects on human health. Nutr Res Rev 18: 98-112.
- Fredrickson WR, FS Group, Inc (2000) Method and Composition for Antiviral Therapy with Olive Leaves. US Patent 6: 117-884.
- Andreadou I, Iliodromitis EK, Mikros E, Constantinou M, Agalias A, et al. (2006) The olive constituent oleuropein exhibits anti-ischemic, antioxidative, and hypolipidemic effects in anesthetized rabbits. J Nutr 136: 2213-2219.
- Andreadou I, Sigala F, Iliodromitis EK, Papaefthimiou M, Sigalas C, et al. (2007) Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. J Mol Cell Cardiol 42: 549-558.
- Rigacci S, Guidotti V, Bucciantini M, Parri M, Nediani C, et al. (2010) Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J Nutr Biochem 21: 726-735.
- Rigacci S, Guidotti V, Bucciantini M, Nichino D, Relini A, et al. (2011) Aß(1–42) aggregates into non-toxic amyloid assemblies in the presence of the natural polyphenol Oleuropein aglycon. Curr Alzheimer Res 8: 841-852.
- Wu C, Lei H, Wang Z, Zhang W, Duan Y (2006) Phenol red interacts with the proto?bril-like oligomers of an amyloidogenic hexapeptide nfgail through both hydrophobic and aromatic contacts. Biophys J 91: 3664-3672.
- Link CD (1995) Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans, Proc Natl Acad Sci USA 92: 9368-9372.
- Grossi C, Rigacci S, Ambrosini S, Ed Dami T, Luccarini I, et al. (2013) The polyphenol oleuropein aglycone protects TgCRND8 mice against Aß plaque pathology. PLoS One 8: e71702.
- Kostomoiri M, Fragkouli A, Sagnou M, Skaltsounis LA, Pelecanou M, et al. (2013) Oleuropein, an anti-oxidant polyphenol constituent of olive promotes a-secretase cleavage of the amyloid precursor protein (AßPP). Cell Mol Neurobiol 33: 147-154.
- Pitt J, Roth W, Lacor P, Smith AB III, Blankenship M, et al. (2009) Alzheimer’s-associated Aß oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol Appl Pharmacol 240: 189-197.
- Soares ML, Coelho T, Sousa A, Batalov S, Conceição I, et al. (2005) Susceptibility and modifier genes in Portuguese transthyretin V30M amyloid polyneuropathy: complexity in a single-gene disease. Hum Mol Genet 14: 543-553.
- Holmgren G, Ericzon BG, Groth CG, Steen L, Suhr O, et al. (1993) Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet 341: 1113-1116.
- Ferreira N, Saraiva MJ, Almeida MR (2011) Natural polyphenols inhibit different steps of the process of transthyretin (TTR) amyloid fibril formation. FEBS Lett 585: 2424-2230.
- Ferreira N, Saraiva MJ, Almeida MR (2012) Epigallocatechin-3-gallate as a potential therapeutic drug for TTR-related amyloidosis: "in vivo" evidence from FAP mice models. PLoS One 7: e29933.
- Ferreira N, Cardoso I, Domingues MR, Vitorino R, Bastos M, et al. (2009) Binding of epigallocatechin-3-gallate to transthyretin modulates its amyloidogenicity. FEBS Lett 583: 3569-3576.
- Azevedo E, Silva FP, Palhano F, Braga CA, Foguel D (2013) Transthyretin-Related Amyloidoses: A Structural and Thermodynamic Approach. In: Dali Feng Amyloidosis, InTech, Croatia.
- Pawar AP, Dubay KF, Zurdo J, Chiti F, Vendruscolo M, et al. (2005) Prediction of ''aggregation-prone'' and ''aggregation-susceptible'' regions in proteins associated with neurodegenerative diseases. J Mol Biol 350: 379-392.
- Porat Y, Mazor Y, Efrat S, Gazit E (2004) Inhibition of islet amyloid polypeptide fibril formation: a potential role for heteroaromatic interactions. Biochemistry 43: 14454-14462.
- Gazit E (2002) A possible role for p-stacking in self-assembly of amyloid fibrils. FASEB J 16: 77-83.
- Sekijima Y, Kelly JW, Ikeda S (2008) Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr Pharm Des 14: 3219-3230.
- Leri M, Nosi D, Natalello A, Porcari R, Ramazzotti M, et al. (2016) The Polyphenol Oleuropein aglycone hinders the growth of toxic Transthyretin amyloid assemblies. J Nutr Biochem under press.
- Daccache A, Lion C, Sibille N, Gerard M, Slomianny C, et al. (2011) Oleuropein and derivatives from olives as Tau aggregation inhibitors. Neurochem Int 58: 700-707.
- Obici L, Merlini G (2014) An overview of drugs currently under investigation for the treatment of transthyretin-related hereditary amyloidosis. Expert Opin Investig Drugs 23: 1239-1251.
- Miyata M, Sato T, Kugimiya M, Sho M, Nakamura T, et al. (2010) The crystal structure of the green tea polyphenol (-)-epigallocatechin gallate-transthyretin complex reveals a novel binding site distinct from the thyroxine binding site. Biochemistry 49: 6104-6114.
- Calamai M, Pavone FS (2013) Partitioning and confinement of GM1 ganglioside induced by amyloid aggregates, FEBS Lett 587: 1385-1391.
- Bucciantini M, Nosi D, Forzan M, Russo E, Calamai M, et al. (2012 ) Toxic effects of amyloid ?brils on cell membranes: the importance of ganglioside GM1. FASEB J 26: 818-831.
- Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, et al. (2010) Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron 66: 739-754.
- Furukawa K, Ohmi Y, Ohkawa Y, Tokuda N, Kondo Y,et al. (2011) Regulatory mechanisms of nervous systems with glycosphingolipids. Neurochem Res 36: 1578-1586.
- Yu RK, Tsai YT, Ariga T (2012) Functional roles of gangliosides in neurodevelopment: an overview of recent advances, Neurochem Res 37: 1230-1244.
- Hou X, Richardson SJ, Aguilar MI, Small DH (2005) Binding of amyloidogenic transthyretin to the plasma membrane alters membrane fluidity and induces neurotoxicity. Biochemistry 44: 11618-11627.
- Pieri L, Madiona K, Bousset L, Melki R (2012) Fibrillar a-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys J 102: 2894-2905.
- Hou X, Mechler A, Martin LL, Aguilar MI, Small DH (2008) Cholesterol and anionic phospholipids increase the binding of amyloidogenic transthyretin to lipid membranes. Biochim Biophys Acta 1778: 198-205.