- (2008) Volume 9, Issue 1
Monica Pandolfi, Margareth Martino, Armando Gabbrielli
Digestive Diseases Department, Campus Biomedico University. Rome, Italy
Tumors of the papilla of Vater are rare, with a prevalence of 0.04%±0.12% reported in autopsy studies. They may occur as sporadic lesions or in patients with familial adenomatous polyposis. Histologically, the malignant transformation of benign adenomas to carcinomas has been documented. The reported frequency of malignancy in an adenoma of the papillae ranges from 26 to 65%. Adenomatous residual foci, as well as areas of moderate to severe dysplasia, have also been found in up to 90% of resection specimens of carcinoma of the major papilla. Based on these observations, adenomas of the major or the minor papilla are thought to exhibit the same adenoma-carcinoma sequence as adenomas of the colon [1].
For these reasons, complete removal of these lesions is mandatory, but the treatment of choice remains controversial.
Surgery represents the traditional option, including pancreaticoduodenectomy which has a peri-operative mortality rate ranging from 2 to 9% and a very significant postoperative complication rate (up to 41% in a large series) [2] and duodenotomy with local excision; the latter is certainly less invasive, but it is associated with local recurrence.
Thus, because of low morbidity and mortality, endoscopic treatment has gained increasing consensus as a treatment of first choice, even if the difficult anatomic location of these lesions makes resection a much more complex procedure as compared to a polypectomy in the colon [3]; furthermore, subsequent surgery in operable patients is not precluded by previous endoscopic resection [4].
On the basis of endoscopic appearance alone, ampullary adenomas cannot always be distinguished from ampullary carcinomas or non-adenomatous polyps (carcinoid tumors, gangliocytic paragangliomas, etc.).
Thus, a definitive tissue diagnosis is a prerequisite for appropriate management, but forceps biopsies in a certain percentage of cases do not allow a correct histological determination of the lesion.
To overcome this difficulty some authors propose a more extensive diagnostic and therapeutic use of ampullectomy instead of a forceps biopsy: the quality of the histological specimens may be better, the pathological diagnosis more accurate and the need for new biopsies significantly reduced [5].
Another important focus of staging is represented by the evaluation of the biliary and/or pancreatic intraductal growth which has been considered to be a contraindication for endoscopic therapy by many authors; therefore, endoscopic retrograde cholangiopancreatography (ERCP) with a side-viewing duodenoscope is required in all patients in order to obtain both a cholangiogram and a pancreatogram before resection to demonstrate a potential intraductal extension of the tumor.
In addition to ERCP, endoscopic ultrasound (EUS) and/or intraductal ultrasound (IDUS) can be performed to provide more detailed and accurate information on the extent of the papillary lesion, such as the size and the echogenicity of the tumor, layered structures of the duodenal wall and regional lymph node status. The data reported in the literature have revealed that linear EUS is superior to helical CT in the preoperative assessment of tumor size, detection of regional nodal metastases and detection of major vascular invasion in patients with periampullary malignancies. Linear array EUS has improved the preoperative local staging of periampullary malignancies when cases were compared by findings at surgery (Tables 1 and 2).
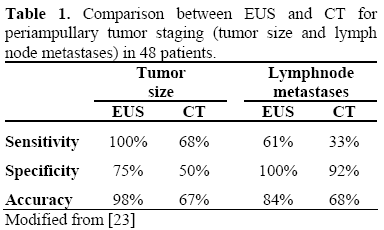
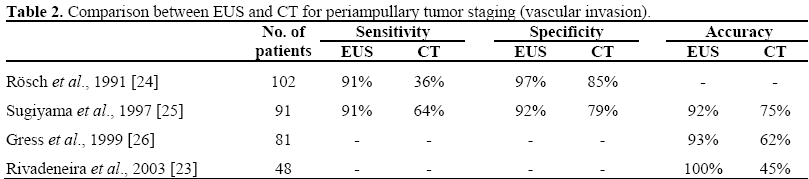
There is no consensus as to which ampullary adenomas should be kept under surveillance and which lesions should be removed endoscopically or surgically.
Several authors have advocated that endoscopic resection should only be performed in patients without evidence of invasive cancer. For other authors, endoscopic resection is not controindicated even in the case of evidence of a high-grade dysplasia.
Table 3 shows the criteria for the endoscopic resectability most frequently reported in the literature. It should be noted that there is a notable variability in inclusion criteria. Some authors exclude lesions greater than 4-4.5 cm for local resection whereas others include them; application of piecemeal resection, when appropriate, has contributed to increasing the size of the lesions treated: for example, tumors up to 7 cm in diameter have been successfully resected.
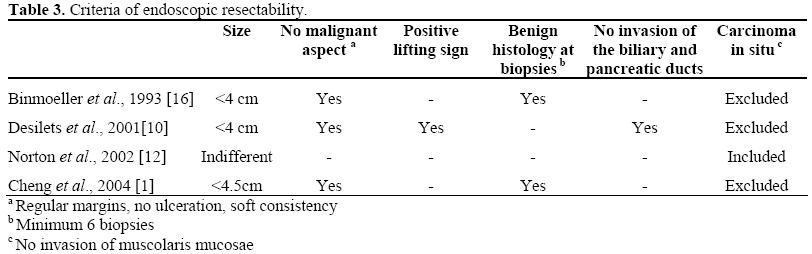
However, the crucial point is represented by the histological staging of ampullary adenomas. Generally, local excision is an accepted curative treatment for an adenoma with high-grade intraepithelial neoplasia/tumor in situ (HGIN/Tis). In T1 cancer, defined according to the TNM classification as a tumor limited to the ampulla or the sphincter of Oddi, there is lymphovascular invasion or lymph-node metastases and ductal involvement in 10-50% and 20-40% of cases, respectively. Therefore, in this case, there is a greater chance of incomplete resection and/or cancer recurrence; similar to the management of colorectal adenomas, endoscopic ampullectomy may be curative for T1 cancer without lymphovascular invasion if histological examination of the entire resected specimen confirms complete removal [6].
Furthemore, another impotant focus regards the intraductal growth, which can be found in both Tis and T1 adenomas and also in adenomas with low grade displasia. Bohnacker et al. [7] suggest that surgery is recommended in the case of intraductal growth in Tis and T1; in adenomas with low grade dysplasia, if the intraductal growth is smaller than 1 cm, there is still a choice of endoscopic resection. In any case, surgery remains the only choice in the case of incomplete removal and if malignancy is clearly present [3].
Figure 1 shows a possible flow-chart for orienting the therapeutic steps.
In the presence of familial adenomatous polyposis, some recommendations must be made. These patients very often develop adenomas in the second part of the duodenum, including the papilla of Vater, but pancreaticoduodenectomy is not always mandatory.
The risk of cancer is assessed by the Spigelman classification (Table 4) [8]. About 10-30% of patients with familial adenomatous polyposis develop a Spigelman stage IV duodenal adenomatosis; mutations downstream from codon 1051 seem to be associated with severe periampullary lesions [9]. These patients have a cumulative cancer risk of 30-40% and a prophylactic pancreaticoduodenectomy should therefore be considered.
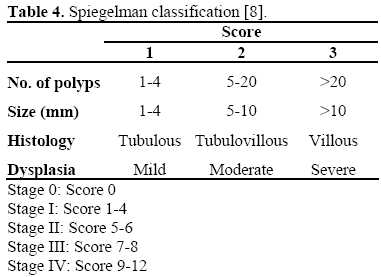
Endoscopic treatment can be performed in the remaining stages (eventually associated with chemoprevention in stages II-III) with a close endoscopic follow-up after the complete excision of the ampullary lesion [3, 9].
Techniques for the endoscopic removal of ampullary adenomas remain nonstandardized, probably due to the relatively small number of procedures of this type It is important to remember that the term ‘‘ampullectomy’’ refers to the removal of the entire ampulla of Vater and it is a surgical term for procedures which require surgical reimplantation of the distal common bile duct and pancreatic duct within the duodenal wall.
Technically, when endoscopic resections of lesions at the major papilla are performed, only tissue from the papilla can be removed endoscopically, and thus the term ‘‘papillectomy’’ is more appropriate than the term ‘‘ampullectomy’’ although the two are often used interchangeably in the literature.
The duodenal papillae (major and minor) should be inspected using a side-viewing endoscope (duodenoscope), as papillary pathology is easily missed with forwardviewing instruments. A submucosal injection prior to the resection is recommended by some authors [7, 10], but not routinely required (similar to that used before performing endoscopic mucosal resection for colorectal polyps). The failure of a lesion to manifest a ‘‘lift sign’’ is associated with malignancy and is considered by many authors as a contraindication to attempts at complete endoscopic resection.
On the contrary, some investigators do not recommend submucosal injection because it can be difficult to capture the lesion with a snare and because submucosal injection may blur the margin of the tumor and does not elevate the bile duct which runs through the duodenal wall.
Endoscopic papillectomy is performed by use of endoscopic snares and electrocautery. In most reports, standard ‘‘braided’’ polypectomy snares have been used, although fine-wire snares specifically designed for ampullary resection are available. There is no evidence documenting the advantage of one type of snare over another.
If the lesion can be completely ensnared, en bloc resection can be performed. En bloc resection has the advantage of potentially shortening the procedure time, requiring less electrocautery, and providing a complete tissue sample for pathologic evaluation. However, piecemeal resection is often performed for lesions larger than 2 cm, in cases where an attempt at en bloc resection has left visible neoplastic tissue in place or to minimize the risk of perforations. Piecemeal resection may produce electrocautery-related injury to tissue fragments sent for pathologic analysis. Larger lesions may require multiple endoscopic procedures in order to be completely removed. Most published series have reported using a combination of en bloc and piecemeal resection techniques as the types of lesions treated were of mixed size and structure. There is no consensus as to which type of current should be used during endoscopic papillectomy. Both pure cutting current, blended current or endocut have been used and neither has been proven to be superior over the other at this time. Power settings are also not standardized [1, 10, 12].
After papillectomy (en bloc or piece-meal) Nd:YAG laser or argon plasma coagulation could be used to remove remaining adenomatous tissue [13]. Pancreatic or biliary sphincterotomy may assist in providing pancreaticobiliary drainage after papillectomy and may simplify attempts to access the common bile duct and pancreatic duct for stent placement. They may also help in postprocedure endoscopic surveillance.
Several studies have shown that placement of a prophylactic pancreatic duct stent reduces the risk of post-ERCP pancreatitis [10, 14, 15], minimizing the risk of stenosis of the pancreatic duct orifice and may also allow safer use of adjunctive coagulative therapies, but this theory is unproven. Others advocate pancreatic stent placement only if the pancreatic duct does not drain after papillectomy [16, 17].
In the study of Zádorová et al. [17], the rate of pancreatitis in patients who underwent papillectomy with and without a pancreaticduct stent was 0% and 20%, respectively. A multicenter study [12] found post-procedure pancreatitis to be more common in patients who did not have a pancreatic stent placed (17% vs. 3.3%), although the difference was not statistically significant. In the study of Cheng et al. [1], prophylactic placement of a pancreatic stent was associated with a lower rate of post-ESP pancreatitis (9.6% vs. 25%; P=0.33) [18, 19].
However, the only prospective, randomized, controlled trial to evaluate the role of prophylactic pancreatic duct stenting for the reduction of post-ERCP pancreatitis after endoscopic papillectomy showed a statistically significant decrease in the rate of postprocedure pancreatitis in the stent group [20]. On the basis of these data, prophylactic pancreatic duct stenting during papillectomy is recommended to reduce the risk of postprocedural pancreatitis whereas .there is no data as to how long the duct should be stented [20].
Although there is extensive discussion about pancreatic duct stent placement, there is also a question regarding the need for biliary drainage after a papillectomy. There have been occasional reports of biliary stent placement after endoscopic papillectomy [1, 16], with the diameter of the stent used varying from 7F to 10F. Nasobiliary drainage could be a valid alternative to a stent permitting X-ray control of the drainage of the bile duct for a few days after the procedure without the need for a new endosocopic examination.
Theoretically, cholangitis can occur after endoscopic papillectomy by the same pathogenetic mechanism as post-papillectomy pancreatitis and, in effect, a case of cholangitis after endoscopic papillectomy has been reported [21]. In any case prophylactic biliary stenting to reduce the risk of postprocedural cholangitis has not been widely performed and cannot be uniformly recommended at this time unless there is concern about inadequate biliary drainage after a papillectomy.
Endoscopic removal of ampullary adenomas is considered a ‘‘high-risk’’ procedure for complications, with a morbidity and mortality of 23% (range 10-58%) and 0.4% (range 0- 7%), respectively. Complication rates reported by large, tertiary care referral centers and experienced therapeutic endoscopists are as follows: pancreatitis 8 to 15%, perforation 0 to 4%, bleeding 2 to 13%, cholangitis 0 to 2% and papillary stenosis 0 to 8%. Therefore, a period of postprocedure inpatient observation should be considered for the detection and treatment of any immediate or slightly delayed complications, especially after extensive removal and treatment of large lesions, in patients with comorbid medical illnesses, those who do not have ready access to medical care and those without support measures.
Most cases of bleeding reported in the literature refer to procedural bleeding and have been managed endoscopically during the same procedure. The consensus of opinion is that these should not be regarded as complications. Delayed bleeding has been reported in approximately 3% of cases and was usually also managed conservatively or by endoscopic re-intervention. Only a few cases of severe pancreatitis requiring prolonged hospitalization have been reported. Surgery is rarely required [20].
Late complications include the development of pancreatic or biliary stenosis; these complications seem more frequent in patients without pancreatic duct stents placed after papillectomy (15.4%) with respect to patients who received pancreatic stents (1.1%) [15]. Some authors [10, 22] suggest that it is possible to reduce the complication rate with routine biliary and pancreatic sphincterotomy, placement of a pancreatic stent before resection, submucosal injection of epinephrine and piece-meal resection in larger lesions.
Results of endoscopic treatment of ampulla tumors reported in the literature are shown in Table 5. It should be noted that, in the study of Catalano et al., the success rate was associated with older age (54.7 vs. 46.6 years; P=0.08), smaller lesions (21.1 vs. 29.7 mm; P<0.0001) and sporadic lesions (63 of 72 vs. 20 of 31; P=0.02) [15].
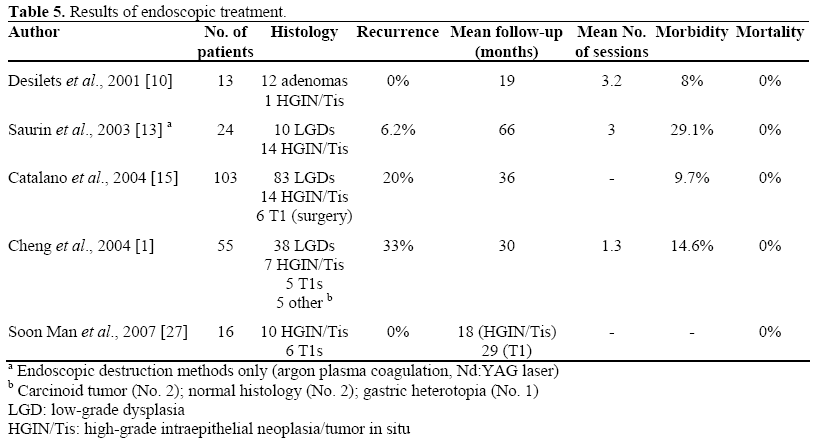
Bohnacker et al. [7] focalized the results on the presence or absence of intraductal tumor growth; in their report carried out on 106 patients studied for a median follow-up of 43 months, the authors found that endoscopic resection was curative in 83% of patients without intraductal growth and in 46% of patients with intraductal growth (P<0.001), concluding that endoscopic ampullectomy is safe and effective, and may be feasible in cases of intraductal growths less than 1 cm.
In the study of Cheng et al., [1], six patients (out of the 55 patients enrolled) had intraductal growths: two underwent surgical resection, considered to be the treatment of choice; four patients (poor candidates for surgery or refusing surgery) underwent intraductal therapy with Nd:YAG laser (2 patients) or endoscopic snare resection (2 patients). In these four patients, the intraductal tumor was eliminated although one of the patients who had laser therapy had a recurrence at a mean follow-up of 12 months. Therefore, the authors suggest that, in patients not eligible for surgery, intraductal adenoma can be eradicated also without considering the cut-off indicated by Bohnacker [7] (tumor extending less than 1 cm from the biliary orifice).
Recurrence rate reported in the literature ranges from 0 to 33%; predisposing factors seem referable to larger size and genetic predisposition; the application of adjuvant thermal ablation can reduce the recurrence rate [15] even if this technique is also associated with an increasing morbidity [13]. However, very often the recurrent tissue is limited in size, histologically benign, without intraductal growth and thus easily amenable to endoscopic techniques [1].
Surveillance after Endoscopic Treatment
An optimal program of surveillance after complete excision of ampullary tumor is not standardized. Based on the experiences reported in the literature, it seems desirable - once complete excision is achieved - to perform ERCP with biopsies of the ampullary region every 6 months for 2 years.
In cases where there is no recurrence after 2 years, endoscopic surveillance should be carried out more frequently in patients with familial adenomatous polyposis than in patients with sporadic lesions because of the high prevalence of duodenal adenomas and the risk of periampullary cancer. Therefore, patients with familial adenomatous polyposis should undergo endoscopy at 3 year intervals whereas the remaining patients should repeat endoscopy only in presence of symptoms [3, 7, 15].
Endoscopic treatment of adenomas of the major duodenal papilla is a safe, welltolerated alternative to surgical therapy. In expert hands, complications are rare and surgery is generally not required. The eligible patients must be selected carefully and an endoscopic follow-up must be assured. A longer follow-up is needed to determine the true recurrence rate (33% in the literature) and the long-term results of endoscopic retreatment [1].
The authors have no potential conflicts of interest