- (2007) Volume 8, Issue 5
Siriboon Attasaranya, Shireen Pais, Julia LeBlanc, Lee McHenry, Stuart Sherman, John M DeWitt
Department of Medicine, Division of Gastroenterology and Hepatology, Indiana University School of Medicine. Indianapolis, IN, USA
Received May 30th, 2007 - Accepted June 21st,. 2007
Context Endoscopic ultrasound (EUS) with EUS-guided fine needle aspiration (EUSFNA) has been increasingly utilized to differentiate malignant/pre-malignant pancreatic cysts from those that are benign or have low malignant potential. Objective To determine the utility of EUS morphology, EUS-FNA cytology and cyst fluid analysis to distinguish mucinous cystic neoplasms from non-mucinous cystic neoplasms based on histopathology following surgical resection. Design A retrospective, single center case series. Participants Patients who underwent EUS and EUS-FNA of known or suspected pancreatic cysts followed by surgical resection. The final diagnosis was based on histopathology. Setting Patients were divided in two groups: mucinous cystic neoplasms and nonmucinous cystic neoplasms. Patients with intraductal papillary mucinous tumors were excluded. Main outcome measures Clinical profiles and EUS findings. Results Forty-eight patients (mean age: 52 years; 29 females, 19 males) were identified: 16 mucinous cystic neoplasms and 32 nonmucinous cystic neoplasms. There were more women in the mucinous cystic neoplasm group compared to the non-mucinous cystic neoplasm group (88% vs. 47%; P=0.011) but the two groups were otherwise similar. The sensitivity, specificity and frequency of cases correctly identified of EUS-FNA cytology for the diagnosis of mucinous cystic neoplasms were 12.5% (95% CI: 2.2-37.2%), 90.6% (95% CI: 75.0-97.5%) and 64.6% (95% CI: 50.4-77.0%), respectively. Median cyst fluid CEA for the mucinous cystic neoplasm group (277 ng/mL; n=14) was significantly higher (P=0.002) than the non-mucinous cystic neoplasm group (1.5 ng/mL; n=21). Cyst fluid CEA greater than 800 ng/mL had a sensitivity of 42.9% (95% CI: 21.3-67.4%) and specificity of 95.2% (95% CI: 75.6-99.9%) for the diagnosis of mucinous cystic neoplasm. On the other hand, a cyst fluid CEA greater than a best cut-off ranging from 3.5 to 8.5 ng/mL had a sensitivity of 92.9% (95% CI: 66.5-100%), a specificity of 66.7% (14/21; 95% CI: 45.2-83.0%), and an accuracy of 81.1% with a frequency of cases correctly identified of 77.1% (95% CI: 60.7- 88.2%). Conclusions EUS-FNA cytology and cyst fluid CEA greater than 800 ng/mL are insensitive but highly specific for differentiating mucinous cystic neoplasms from nonmucinous cystic neoplasms. EUS morphology alone cannot distinguish between the two groups.
Biopsy, Fine-Needle; Endosonography; Pancreatic Cyst
CA 19-9: carbohydrate antigen 19-9; IPMN: intraductal papillary mucinous neoplasm; MCN: mucinous cystic neoplasm; NMCN: non-mucinous cystic neoplasm; NPV: negative predictive value; SCA: serous cystadenoma; PNT: pancreatic neuroendocrine tumor; PPV: positive predictive value
Pancreatic cysts are often initially identified by magnetic resonance imaging (MRI) or computed tomography (CT) during evaluation of varied symptoms such as abdominal pain, pancreatitis, weight loss, or jaundice. In fact, up to 37% of these lesions may be discovered incidentally [1]. Pancreatic cysts represent a wide clinicopathological spectrum and are generally classified into mucinous cystic neoplasms (MCNs), non-mucinous cystic neoplasms (NMCNs) and pseudocysts. Overall, pseudocysts comprise about 80% of documented pancreatic cysts, whereas pancreatic cystic neoplasms account for the remaining 20% [2, 3]. As nonepithelialized cysts, pseudocysts have no malignant potential. NMCNs which include (among others) serous cystadenomas, simple cysts, and cystic pancreatic neuroendocrine tumors are considered to have little or low malignant potential. With the exception of pancreatic neuroendocrine tumors, NMCNs are generally managed nonoperatively in the absence of symptoms or complications [4, 5]. MCNs include mucinous cystadenomas, mucinous cystadenocarcinoma, and intraductal papillary mucinous neoplasms (IPMNs). As these have an increased risk of malignant degeneration, these cysts are often managed surgically in appropriate candidates [4, 5]. Differentiating NMCNs from MCNs remains a clinical challenge and currently no single clinical parameter or imaging finding reliably distinguishes these two groups [6].
Endoscopic ultrasound (EUS) has been increasingly used as a supplementary test to CT and MRI for the evaluation of pancreatic cystic lesions. The reported accuracy of EUS morphology alone for differentiating benign from pre-malignant or malignant pancreatic cysts ranges 40-93% [7, 8]. The addition of EUS-guided fine-needle aspiration (EUSFNA) permits cyst fluid sampling and analysis for cytologic evaluation and tumor markers. EUS-FNA cytology has a reported sensitivity of 22-95% for diagnosis of pancreatic cystic tumors [9, 10, 11, 12]. Similarly, pancreatic cyst fluid markers have a wide range of diagnostic accuracies for the classification of these lesions [10, 12, 13, 14, 15, 16]. One recent pooled analysis demonstrated that cyst fluid CEA greater than 800 ng/mL had a sensitivity of 48% and specificity of 98% for the diagnosis of MCNs [17]. The purpose of our single center retrospective case series is to examine the utility of EUS characteristics, EUS-FNA cytology, and fluid analysis to distinguish MCNs from NMCNs in patients who underwent surgical resection. Patients with IPMNs were excluded from this study.
Patient Population
Using prospectively updated endoscopy, cytology and surgery databases, patients at Indiana University who underwent EUS and EUS-FNA of known or suspected pancreatic cysts followed by surgical resection from 1996-2005 were identified. Medical records, endoscopy and operative reports, and pathology reports were reviewed. Demographic data, symptom onset, presenting symptoms, any history of pancreatitis, results of previous imaging studies, EUS morphology, and cyst fluid analysis from EUS-FNA including cytology, carcinoembryonic antigen (CEA), carbohydrate antigen (CA 19-9), amylase and lipase were recorded. Using surgical histopathology as the gold standard, pancreatic cystic lesions were classified as pseudocysts, MCNs and NMCNs. Patients with intraductal papillary mucinous tumors were excluded from this study due to analysis in a separate report.
Interventions
Patients received conscious sedation with various combinations of intravenous midazolam, meperidine, fentanyl, or propofol under appropriate cardiorespiratory monitoring. All procedures were performed by or under the supervision of one of seven experienced attending physicians. EUS examinations were usually initiated with a radial echoendoscope (GF-UM20, GFUM- 130, or GF-UM160, Olympus Corporation, Melville, NY, USA). Curvilinear array endosonography was performed using Pentax or Olympus endoscope (32-UA, 36-UX, Pentax Precision Instruments, Orangeburg, NY, USA; GF-UC30P, GF-UC140P, Olympus Corporation, Melville, NY, USA). EUS-FNA was done using a 22-gauge, 8 cm needle (EUSN-1, EUSN-2, EUSN-3, or Echotip Ultra, Cook Medical Inc., Winston- Salem, NC, USA; EZ-Shot, Olympus Corporation, Melville NY, USA). Doppler color angiography was used to ensure the absence of intervening vascular structures along the anticipated needle path. The site of the EUS-FNA was performed at the discretion of the endosonographer. However, our protocol has been to first to perform one or more passes into any solid component associated with a cystic pancreatic lesion. If no solid component is present then one pass was made into the largest accessible cyst. Suction is applied using a syringe placed onto the proximal end of the needle and the cyst is then aspirated as much as possible.
A cytotechnologist or cytopathologist was available on-site for preliminary interpretations on all procedures. Samples aspirated were expressed onto a glass slide and two smear preparations were made. One slide was airdried and stained with a modified Giemsa stain for rapid on-site interpretation. The other slide was alcohol-fixed and stained by the Papanicolau method. If at least 1 mL of cyst fluid was still available after preliminary cytology interpretation was performed, this additional fluid was sent for measurement of CEA, CA 19-9 or amylase at the discretion of the endosonographer. All patients who underwent EUS-FNA received one dose of intravenous antibiotics in the recovery area prior to discharge. Oral antibiotics were subsequently prescribed for an additional 3-7 days.
Per department policy, all patients were telephoned within 48 hours after the procedure to assess for any short-term complications. For study purposes, intraprocedural complications were defined as: hypotension (absolute SBP less than 80 mmHg); hypoxia (SpO2 less than 85%); bradycardia (less than 50 beats per minute); need for supplemental oxygen above baseline supplementation, positive pressure ventilation or endotracheal intubation, bleeding recognizeed during EUS or by subsequent imaging study, blood transfusion, abdominal pain or requirement for hospitalization.
Cytology Examination
Within several days of each EUS exam, a final cytologic diagnosis was rendered by a staff cytopathologist. Mucin stains were not performed on any specimens. Cytology criteria for the diagnosis of a serous cystadenoma included clusters or sheets of cuboidal epithelial cells with small round nuclei. Benign MCNs were considered to have sheets of bland mucinous epithelial cells and background extracellular mucin. Malignant degeneration of MCNs was considered present when epithelial cells had several of the following features: nuclear pleomorphism, mitoses, prominent nucleoli, three-dimensional arrangements, irregular nuclear membranes and background necrosis [18]. Pancreatic neuroendocrine tumors were considered diagnostic cytologically when uniform, plasmacytoid appearing cells with a round, eccentric nucleus and fine chromatin stained positive for neuroendocrine tumor markers [19]. The results of the final report and immunocytochemistry (if performed) were recorded. Cytology reports were categorized as diagnostic for malignancy, suspicious for malignancy, atypical, benign or nondiagnostic for malignancy. The final diagnosis in each patient was made by the results of surgical resection and corresponding histopathology.
This study was approved by the Institutional Review Board of Indiana University/Clarian Health Partners. All patients gave written informed consent.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS 12.0: SPSS Inc., Chicago, IL, USA). Continuous variables were described with means and standard deviations; median andrange values were also reported. Categorized variables were expressed as absolute and relative frequencies; the 95% confidence intervals (CIs) of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and frequency of cases correctly classified were also computed by using GraphPad software (https://www. graphpad.com/quickcalcs/ConfInterval1.cfm) [20]. Baseline patient characteristics, EUS morphology, and EUS-FNA were compared between the two groups by using the Mann- Whitney U (continuous data), Fisher’s exact (dichotomous data), and Pearson chi-squared (categorical data) tests. A two-tailed P value less than 0.05 was considered statistically significant. Receiver operating characteristic (ROC) curves and the respective areas under the curves (AUC), together with the standard errors (SE), were calculated for cyst fluid CEA, amylase as well as lipase in order to provide more accurate information about the capacity of these tests in distinguish MCNs from NMCNs and pseudocysts from other cystic lesions, respectively. The best cut-off value of the ROC analysis was chosen as the value which maximizes the likelihood ratio (LR) obtained using the following formula: LR = (Probability of true positive + Probability of true negative) / (Probability of false positive + Probability of false negative) [21].
Forty-eight patients (29 women, 19 men; mean age 52±13 years) were identified. A complete medical record was available in 43 (89.6%) patients. Abdominal pain, the most common presenting symptom, was found in 31 (64.6%) patients. Nine (18.8%) patients were asymptomatic. Ten out of 43 (23.3%) had a history of acute or recurrent pancreatitis. The median time from onset of symptoms to EUS was 5 months (range: 0.2- 216 months). The median time between EUS and surgery for all 48 patients was 47 days (range: 5-589 days). Final histopathological diagnoses were classified as a MCN in 16 patients: 13 mucinous cystadenomas and three mucinous cystadenocarcinomas. The remaining 32 non-mucinous cystic neoplasms (NMCNs) included: 14 pseudocysts, 10 serous cystadenomas, three pancreatic neuronendocrine tumors, two lymphoepithelial cysts, two simple cysts and one cystic lymphangioma. EUS-FNA (mean 2.5±1.9 passes) were performed in all 48 patients without known complications. The mean size of cysts was 39±22 mm (median 35 mm; range: 7-120 mm). A cytologic examination of the EUSFNA specimen was performed in all 48 patients. Analysis of cyst fluid CEA, amylase and lipase was performed in 35 (72.9%), 31 (64.6%) and 28 (58.3%) patients, respectively.
Table 1 shows the clinical characteristics and EUS morphology in the two groups of patients. There were significantly (P=0.011) more women with MCNs (87.5%) compared with those with NMCNs (46.9%). However, the age, onset of symptoms, presenting symptom of abdominal pain, antecedent pancreatitis, and number of incidentally identified pancreatic cysts were not significantly different between the two groups. A history of pancreatitis (P=0.010) and EUS evidence of chronic pancreatitis (P=0.015) were more frequent in patients with pseudocysts (6/11, 54.4% and 7/10, 70.0%, respectively) compared to all other patients (4/32, 12.5% and 5/25, 20.0%, respectively). There was no significant difference in EUS cyst morphology and cyst fluid characteristics between the two groups.
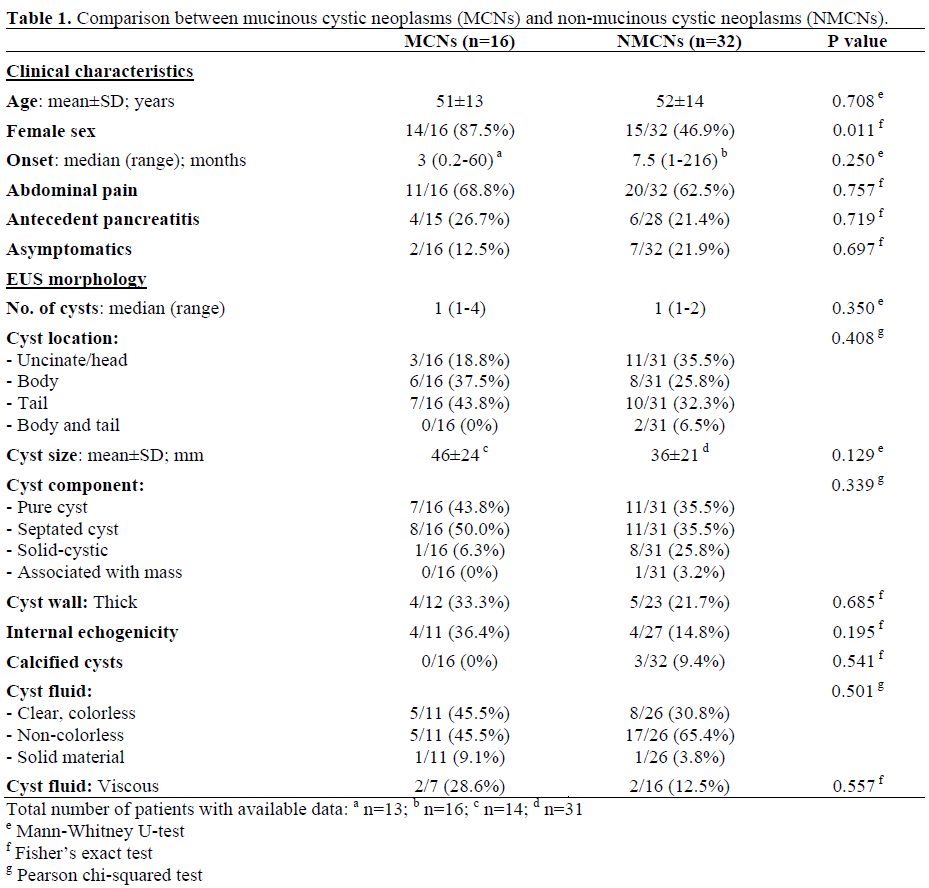
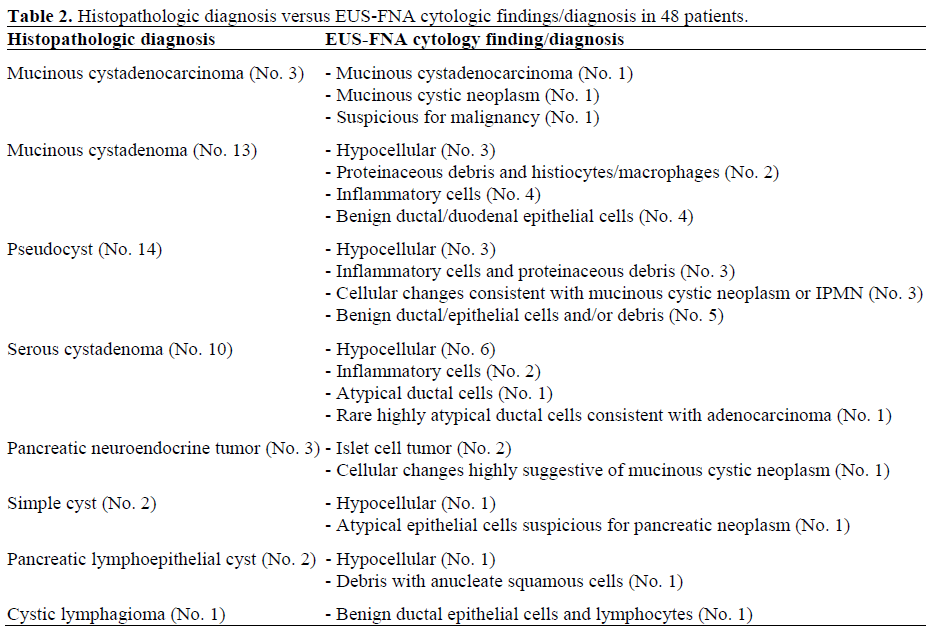
Histologically, extracellular mucin was identified in only three patients (one with MCN, and two with pseudocysts). All had EUS-FNA performed via a transgastric approach. The results of EUS-FNA cytology compared to the corresponding histopathologic diagnosis in all 48 patients are shown in Table 2. Compared to surgical pathology, EUS-FNA cytology provided the correct pathologic diagnosis in 5/48 (10.4%), including two MCNs, two pancreatic neuroendocrine tumors and one pseudocyst. For the three patients with mucinous cystadenocarcinomas, EUSFNA demonstrated mucinous cystadenocarcinoma in one, MCN in one and was suspicious for malignancy in one. EUS-FNA cytology provided the correct diagnosis in two of three patients with pancreatic neuroendocrine tumors. For the cytologic diagnosis of MCNs, EUS-FNA had a sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and frequency of cases correctly classified of 12.5% (2/16; 95% CI: 2.2-37.2%), 90.6% (29/32; 95% CI: 75.0-97.5%), 40.0% (2/5; 95% CI: 11.6-77.0 %), 67.4% (29/43; 95% CI: 52.4-79.5 %) and 64.6% (31/48; 95% CI: 50.4-77.0%), respectively.
Results from cyst fluid CEA measured in 35 patients (14 MCNs, seven serous cystadenomas, two simple cysts, 11 pseudocysts, one pancreatic neuroendocrine tumor) are shown in Figure 1. Cyst fluid CEA of 14 MCNs (median 277 ng/mL, range 0.5- 144,000 ng/mL) was significantly higher (P=0.002) than that of 21 NMCNs (median 1.5 ng/mL, range 0.5-2,243 ng/mL. A cyst fluid CEA value greater than the arbitrary cutoff of 800 ng/mL provided a sensitivity and specificity for the diagnosis of MCNs of 42.9% (6/14; 95% CI: 21.3-67.4%) and 95.2% (20/21; 95% CI: 75.6-99.9%), respectively. Similarly, a cyst fluid CEA greater than 400 ng/mL provided a sensitivity and specificity of 50.0% (7/14; 95% CI: 26.8- 73.2%) and 90.5% (19/21; 95% CI: 69.8- 98.6%), respectively. The ROC analysis showed a good accuracy (AUC: 0.811±0.075); the best cut-off (LR=3.94) ranged from 3.5 to 8.5 ng/mL with a sensitivity, specificity, PPV, NPV and frequency of cases correctly classified of 92.9% (13/14; 95% CI: 66.5-100%), 66.7% (14/21; 95% CI: 45.2-83.0%), 65.0% (13/20; 95% CI: 43.2-82.0%), 93.3% (14/15; 95% CI: 68.2-100%), and 77.1% (27/35; 95% CI: 60.7-88.2%), respectively. The CEA values measured in the seven serous cystadenomas were all between 0.5-1.5 ng/mL. The mean cyst fluid CEA in all 11 pseudocysts was 106±151 ng/mL (median 40 ng/mL, range: 0.5-463 ng/mL).
Figure 1. Cyst fluid carcinoembryonic antigen (CEA)
in 35 pancreatic cystic lesions (CEA was not measured
in 13 patients with pancreatic cyst who underwent
EUS-FNA).
Orange circles represent two patients with mucinous
cystadenocarcinoma.
MCN: mucinous cystic neoplasm; SCA: serous
cystadenoma; PNT: pancreatic neuroendocrine tumor
Cyst fluid amylase was determined in 31 patients including nine with pseudocysts and 22 without pseudocysts. Median cyst fluid amylase was higher in pseudocysts (19,834 U/L, range: 10,675-146,702 U/L) compared to all other cystic lesions (882 U/L; range: 25- 134,224 U/L) but the difference resulted at the limit of the statistically significance (P=0.050). The ROC analysis showed a good accuracy (AUC: 0.727±0.090) for differentiating a pseudocyst from all other pancreatic cysts. The best cut-off (LR=4.50) ranged from 1,980 to 10,675 U/L with a sensitivity, specificity, PPV, NPV and frequency of cases correctly classified of 100% (9/9; 95% CI: 65.5-100%), 63.6% (14/22; 95% CI: 42.8-80.3%), 52.9% (9/17; 95% CI: 30.9-73.8%), 100% (14/14; 95% CI: 74.9-100%), and 74.2% (23/31; 95% CI: 56.5-86.5%), respectively. The eight patients with cyst fluid amylase greater than 5,000 U/L that were not pseudocysts included seven mucinous cystadenomas and one simple cyst. Cyst fluid lipase was obtained in 28 patients including seven with pseudocysts and 21 without pseudocysts. Median cyst fluid lipase was significantly (P=0.012) higher in pseudocysts (508,000 U/L, range: 60,700- 4,500,000 U/L) compared to all other cystic lesions (1,149 U/L; range: 16-9,880,000 U/L). The ROC analysis showed a good accuracy (AUC: 0.823±0.077) in distinguish pseudocysts from other cystic lesions. The best cutoff (LR=5.00) ranged from 23,961 to 60,700 U/L with a sensitivity, specificity, PPV, NPV and frequency of cases correctly classified of 100% (7/7; 95% CI: 59.6-100%), 66.7% (14/21; 95% CI: 45.2-83.0%), 50.0% (7/14; 95% CI: 26.8-73.2%), 100% (14/14; 95% CI: 74.9-100%), and 75.0% (21/28; 95% CI: 56.4-87.6%), respectively. Of note, two of three mucinous cystadenocarcinomas presented with recurrent pancreatitis and were septated by EUS morphology. The remaining mucinous cystadenocarcinoma was a mixed solid and cystic lesion. Cyst fluid CEA of the two mucinous cystadenocarcinomas without a solid component were 144,000 ng/mL and 5,222 ng/mL. EUS-FNA of the solid-cystic lesion in one remaining patient yielded no cystic fluid for analysis. In these three patients with mucinous cystadenocarcinomas, EUS-FNA cytology was positive for malignant cells in one, suspicious for malignancy in one and mucinous cystic neoplasm with uncertain malignant potential in one.
Differentiating NMCNs from MCNs of the pancreas remains a clinical challenge and currently no single clinical parameter or imaging finding reliably distinguishes these two groups [6]. As an adjunctive tool to other imaging studies, EUS with EUS-FNA has been found to have a wide range of sensitivities for distinguishing MCNs from other pancreatic cystic lesions [10, 12, 13, 14, 15, 16]. We found that compared to surgical pathology, EUS-FNA cytology of pancreatic cysts provided a sensitivity of only 10% for the diagnosis among all 48 patients. The sensitivity and specificity of cytology for the diagnosis of MCNs (n=16) were 13% and 91%, respectively. Among three mucinous cystadenocarcinomas, EUS-FNA cytology provided correct diagnosis in one, suspicious for malignancy in one and diagnosis of MCN in one. However, none of 13 MCNs was correctly diagnosed by EUS-FNA cytology. The poor sensitivity and good specificity of EUS-FNA in our study for the diagnosis of MCNs is similar to that reported by the multicenter Cooperative Pancreatic Cyst Study [10], which found a sensitivity and specificity of 35% and 83%, respectively of EUS-FNA cytology for the diagnosis of MCNs in 112 patients who underwent surgery. Collectively, these data show the limitations of cytology alone for the diagnosis of these lesions. These findings are in contrast to a large single center study by Frossard et al. [12] which found that EUS-FNA cytology correctly diagnosed 65 of 67 (97%) pancreatic cystic lesions. These researchers [12] used an additional cell preparation processor that provided a monolayered cell population with availability of a dedicated cytopathologist. More research is needed to validate these findings.
Our study found that the median cyst fluid CEA in MCNs is significantly higher than that in NMCNs, similar to that reported by others [10]. Furthermore, we found that cyst fluid CEA greater than 800 ng/mL had a sensitivity and specificity of 42% and of 95%, respectively for distinguishing MCNs from other cystic lesions. Based on the high sensitivity and NPV of the best cut-off range (3.5-8.5 ng/mL) derived from the ROC analysis in our study, pancreatic cystic lesions with a low value (less than 10 ng/mL) of cyst fluid CEA are unlikely to be MCNs. In a recent pooled analysis [17], the same cyst fluid CEA cut-off value (greater than 800 ng/mL) demonstrated a sensitivity of 48% and specificity of 98% for differentiating MCNs from other cysts. Other studies evaluating cyst fluid CEA have reported sensitivities of 13- 88% and specificities ranging 44-100% at various cut-off values [10, 12, 13, 14, 15, 16]. While cyst fluid CEA values greater than 800 ng/mL are specific for MCNs, we found that the measured values for MCNs may overlap between benign and malignant tumors. At present, the utility of cyst fluid CEA to predict the presence or absence of malignancy is still unclear [10] and requires further evaluation in a larger number of patients. One recent report showed that cyst fluid DNA analysis for K-ras mutation provided a 91% sensitivity and 93% specificity for predicting malignancy in pancreatic cysts [22]. Future studies on DNA analysis from pancreatic cystic fluid will likely improve the utility of EUS in evaluating these lesions.
For predicting serous cystadenomas, we found that cyst fluid CEA less than 5 ng/mL had a sensitivity, specificity and NPV of 100%, 71% and 100%, respectively. The same cut-off value, however, provided a sensitivity and specificity for predicting pseudocysts of only 46% and 58% respectively. In two other studies [12, 13], the CEA value less than 5 ng/mL had a sensitivity and specificity ranging 54-100% and 77-86% in differentiating serous cystadenomas from other pancreatic cysts. In a pooled analysis of several studies, a CEA less than 5 ng/mL suggested serous cystadenoma and pseudocyst with a sensitivity of 50% and specificity of 95% [17]. Due to the wide-range of reported values, the use of a low CEA for predicting benign or low malignant potential cysts requires further investigation.
Cyst fluid amylase was measured in nine patients with pseudocysts, with all reported values above 10,000 U/L. An arbitrary cut-off value of cyst fluid amylase of 5,000 U/L had a sensitivity, and specificity of 100% and 64% respectively for differentiating a pseudocyst from all other pancreatic cysts. This is similar to the other studies which reported a sensitivity ranging 61-94% and specificity ranging 58-74% at the same cutoff value [12, 13]. However, due to the small numbers of patients and the wide range of cyst fluid amylase and lipase values in our series, the utility of these markers for differentiating the two groups can not yet be determined. Interestingly, seven of the eight patients with non-pseudocysts and cyst fluid amylase greater than 5,000 U/mL in our study had mucinous cystadenomas. Two of these seven mucinous cystadenomas also underwent ERCP, and both had a cyst-duct communication. It is well known that MCNs rarely have such a cyst-duct communication [23]. Few cases of such communicating mucinous cystadenomas with high fluid amylase have been reported in the literature [24, 25, 26]. Le Borgne et al. [26] retrospectively reviewed 398 cases of pancreatic cystic tumors who underwent surgery and observed a cyst-duct communication in the resected specimens of 0.6% of 144 serous cystadenomas, 6% of 150 mucinous cystadenomas and 10% of 78 mucinous cystadenocarcinomas. The true incidence of communicating cysts of MCNs (specifically those with high fluid amylase) was thus probably underestimated. The accuracy of EUS morphology for differentiating premalignant/malignant pancreatic cysts was initially reported at 72-96% [9, 27]. Subsequent studies, however, found that EUS morphology may not be as accurate as the earlier studies found [8, 10]. We also found no statistically significant difference of various EUS morphologic features between the MCN and NMCNs. However, since EUS was performed by seven endosonographers during the study period, these findings may reflect the interobserver variation [8]. Our study showed that antecedent pancreatitis and the morphologic change of chronic pancreatitis by EUS were commonly found and associated with pseudocysts. Therefore, these findings may be helpful to predict the diagnosis of pseudocysts.
Our study showed that with the exception of female gender (found predominantly in MCNs), the clinical parameters assessed were not significantly different between MCNs and NMCNs. In addition, the size of pancreatic cyst was not correlated with symptom presentation, as we found no statistically significant (P=0.240) different cyst size between patients who were asymptomatic (mean: 47±30 mm; 8 patients) and symptomatic (mean 37±19 mm; 37 patients). Two of 13 mucinous cystadenomas were incidentally found in our study; a fact underscored in one retrospective study which found that 17% of 78 asymptomatic patients with pancreatic cystic neoplasms, had in situ or invasive cancer [1].
Although this study evaluated patients with cystic neoplasms who underwent EUS-FNA and subsequent surgery, there are several limitations that merit discussion. First, patients with IPMNs were excluded since these tumors were being evaluated in a separate study. These tumors have similarly been excluded from other analyses [17] and unlike serous cystadenomas, MCN, mucinous cystadenocarcinomas, pseudocysts and other pancreatic cysts, the diagnosis of IPMNs are often easily made by distinguishing features of cyst-duct communication, diffuse or segmental duct dilation, mural nodules and intraductal mucin. Second, cyst fluid tumor markers were not acquired in some patients, thereby limiting conclusions that may be made about its utility in these patients. In our experience, EUS-FNA of pancreatic cysts smaller than 15 mm may obtain only small amount of cyst fluid, which is usually inadequate for any analysis other than cytology. Third, we were unable to fully analyze the diagnostic potential of cyst fluid CA 19-9 because of the small sample size (15 out of 48 cases were available only). This is in part due to our decreased use of this test when preliminary reports from the Cooperative Pancreatic Cyst Study [10] suggested that cyst fluid CA 19-9 was of limited value for differentiating mucinous from non-mucinous tumors. Other studies reported the sensitivity and specificity of cyst fluid of CA 19-9 as 15-75% and 62-90% respectively for distinguishing MCNs from other cystic lesions [12, 13, 14, 15, 16].
In conclusion, EUS-FNA cytology and cyst fluid analysis is a useful adjunct to abdominal imaging for the diagnosis of pancreatic cystic lesions. EUS-FNA cytology and cyst fluid CEA greater than 800 ng/mL are insensitive but highly specific for differentiating MCNs from NMCNs. EUS morphology alone cannot distinguish between the two groups.
The authors have no potential conflicts of interest