- (2009) Volume 10, Issue 3
Jose Celso Ardengh1, Carlos Alberto Malheiros2, Victor Pereira2, Djalma Ernesto Coelho3, Jose Flavio Coelho3, Fares Rahal2
1Endoscopy Unit, Division of Anatomy and Surgery, Ribeirão Preto School of Medicine, University of São Paulo, Hospital 9 de Julho. São Paulo, Brazil
2Department of Surgery of Santa Casa de São Paulo. São Paulo, Brazil
3Department of Surgery, Federal University of Rio de Janeiro. Rio de Janeiro, Brazil
Received March 17th, 2009 - Accepted April 14th, 2009
Context EUS-FNA is increasingly being used in operable pancreatic carcinoma cases identified by CT. Objectives Determine the safety, accuracy and clinical utility of EUS-FNA for T, N and TN staging and vascular injury assessment in proven ductal pancreatic carcinoma. Patients Fifty-two consecutive patients (29 women and 23 men) with histologically ductal pancreatic carcinoma, with an excellent possibility of mass resection assessed by helical computerized tomography, were studied. Mean age was 62.4 years (range: 27-82 years). Tumor locations were in the head (43 cases), body (5 cases) and tail (4 cases) of the pancreas. Mean tumor size from EUS was 3.7 cm (range: 0.8-6.2 cm). Methods We reviewed medical records and abdominal ultrasound, CT, EUS-FNA and the results were compared to surgical and histological findings. Results Ultrasound identified pancreatic abnormalities in 38 out of 52 patients (73.1%): pancreatic mass (25 cases), pancreatic head enlargement (8 cases), dilation of main pancreatic duct (3 cases), pancreatic cyst (1 case) and pancreatic calcification (1 case). CT showed a pancreatic mass (30 cases), pancreatic enlargement (17 cases), pancreatic cystic lesion (2 cases) and pancreatic calcification (1 case) in 50 out of 52 patients (96.2%). EUS-FNA found a clear pancreatic tumor image in all patients (100%). The accuracy of EUS for evaluating portal blood vessels, superior mesenteric artery, T alone, N alone and combined TN staging was 86.5%, 94.2%, 84.7%, 67.3% and 55.8%, respectively. In addition to cytological material from 50 patients, microfragments from 43 patients were sent for histological analysis. Two patients (3.8%) showed minor complications: self-limited bleeding and acute pancreatitis. Conclusions EUS-FNA is safe, and can help gastroenterologists and surgeons make surgical decisions regarding pancreatic carcinoma patients.
Adenocarcinoma /diagnosis; Biopsy, Fine-Needle; Endosonography; Neoplasm Staging; Pancreas /pathology; Tomography, Spiral Computed
Ductal pancreatic carcinoma accounts for 90% of exocrine tumors and its development is insidious, progressive and fatal [1]. In Brazil, it accounts for 10.2% of deaths due to cancer [2].
The mean survival following its diagnosis is from four to eight months and overall five-year survival is less than 1% [3]. Surgical resection for curative purposes increases mean survival to from 17 to 20 months and is the only form of curative treatment [1]. At the time of diagnosis, the resectability rate is approximately 6.4% with a five-year survival rate of 3.5% following pancreaticoduodenectomy [4, 5].
Imaging examinations are important for diagnosis and staging. Ideally, form it should determine the location and size of the tumor, and diagnose any hepatic metastases (an important prognostic factor) there may be in celiac, peripancreatic and periaortic lymphatic nodules. They should also identify any invasion of the superior mesenteric vein and artery, portal vein and splenomesenteric confluence so that the best form of treatment can be chosen [6, 7, 8].
Helical computerized tomography (CT) [9] has been used to predict the resectability of pancreatic carcinoma, based on the degree of invasion of the superior mesenteric vein and artery [10], with the aim of avoiding non-therapeutic laparotomy procedures [11].
Radial scanning endoscopic ultrasonography (EUS) is used to identify and stage pancreatic carcinoma [12] and most studies have shown that its diagnostic precision ranges from 70 to 90% [12, 13, 14]. However, few studies have used linear array endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) alone to evaluate the degree of vascular invasion and T and N. In most clinics, this examination is always performed after identifying and staging the tumors using radial EUS. The aim of using EUS-FNA would only be to obtain material for anatomopathological analysis. This study evaluated the role of EUS-FNA used alone and on a single occasion to determine the T and N categories and degree of vascular invasion. The aim was to compare the preoperative and surgical findings from this technique among patients with pancreatic carcinoma, who had previously been studied using CT, with regard to the possibility of tumor resection.
All patients were studied consecutively during the preoperative period by means of EUS-FNA alone, performed only on that occasion, in the Endoscopy and Echoendoscopy Sector of Hospital 9 de Julho, over the period from January 2000 to December 2002. Our service has been performing an average of 35 to 40 linear array EUS examinations per month since January 1997 and, in approximately 35% of the cases, fineneedle aspiration (FNA) puncture has been necessary. The great majority of the indications in our service relate to the biliopancreatic duct, and these account for approximately 80%. Although the group for the present study included only patients with pancreatic carcinoma, such patients are referred by teams in different Brazilian states and have therefore been treated by different surgical teams
We selected and retrospectively studied only patients with a cytological and/or histological diagnosis of pancreatic carcinoma. We excluded those whose CT scans presented signs of unresectability (Table 1) but included the patients whose CT scans presented doubts or indicated the possibility of the patient’s tumor being resected surgically.

The EUS-FNA always followed the protocol below: a) a sectoral system made by Pentax (models FG 36- UX and FG 38-UX, Pentax Precision Instruments Corp., Orangeburg, NY, U.S.A.) was used, coupled to a Hitachi ultrasound module (models EUB 405 and EUB 515A, Mitsubishi, Conshockon, Philadelphia, PA, U.S.A.);
b) an examination was carried out on the entire pancreas, the portal system (portal vein, superior mesenteric vein, splenic vein and splenomesenteric confluence, superior mesenteric artery), common bile duct, main pancreatic duct, duodenal papillae and liver, thus making it possible to classify the tumors in the T categories and identify lymphatic nodules (N) and, c) finally, all tumors, hepatic metastases and lymphatic nodules with suspicion of infiltration which were identified by means of EUS, underwent FNA in an attempt to diagnose the histological type of tumor. We used the GIP 22G needle system (Medizintechnik GmbH, Grassau, Germany) and the needle system from the Olympus Optical Co. (Melville, New York, NY, U.S.A.).
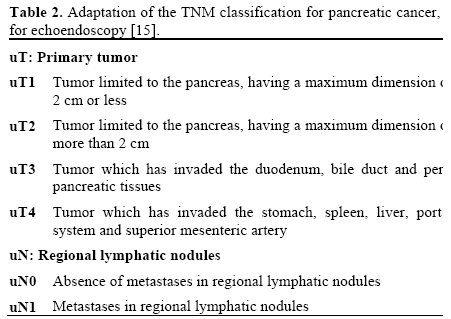
The staging determined by means of EUS was carried out in accordance with the TNM classification (International Union Against Cancer, 1997 [15]), as modified to fit with the interpretation from EUS (Table 2).
T category
We classified all the tumors identified as uT1, uT2, uT3 or uT4 (Table 2). Cases were considered to present vascular invasion by the tumor (uT4), as seen from the EUS, when one or more of the following associated criteria were present: a) absence of a hyperechoic interface; b) irregularity of the vessel wall due to contact with the tumor; c) invasion of the vascular lumen by the tumor; and d) thrombosis of the portal system and the superior mesenteric artery.
N category
Lymphatic nodules were considered to be metastatic when at least four of the following characteristics were found on EUS: a) homogenous texture; b) rounded outline; c) clear limits; d) size greater than 1 cm; and e) the same echo texture as the primary tumor. Such cases underwent FNA.
This study was approved by the Research Ethics Board of Hospital 9 de Julho and written informed consent was obtained from each patient to be included in the study. The study protocol conforms to the ethical guidelines of the "World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects".
We calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy and 95% confidence intervals (95% CI) for the results relating to EUS-FNA, invasion of the portal system and superior mesenteric artery, and N category. The Pearson chi-square and the Fisher’s exact test were used. We used the kappa index for the T and joint TN classifications.
The medical records of 52 patients referred to our institution over a three-year period with a diagnosis of a pancreatic mass and who fulfilled the inclusion criteria were reviewed. In this series, 29 (55.8%) were women and 23 (44.2%) were men, and mean age was 62.4 years (range: 27 to 82 years). The tumors were distributed in the pancreas as follows: 43 (82.7%) in the head, 5 (9.6%) in the body and 4 (7.7%) in the tail. The mean size of the tumors was 3.7 cm (range: 0.8-6.2 cm). The tumors in the body and tail presented a mean size of 4.4 cm (range: 2.5-5.6 cm).
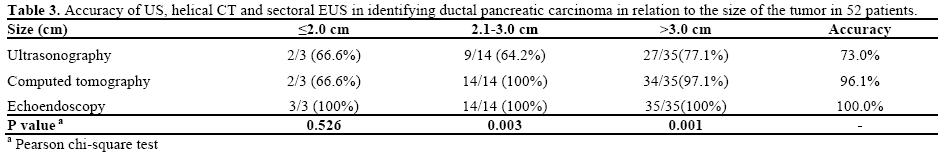
Abdominal ultrasound detected abnormalities in the pancreatic gland in 38 patients (73.1%) and was normal in 14 (26.9%). In the latter, it failed in tumors with sizes between 1.8 and 5.8 cm, of which six were smaller than 3.0 cm. EUS detected all tumors. Comparing the ultrasound and EUS results, statistical significance regarding tumor identification (P<0.001) was found.
CT identified morphological abnormalities in the pancreatic gland in 50 patients (96.2%) and did not identify abnormalities in 2 (3.8%). In the latter cases, ultrasound revealed nodules which CT did not identify. There was no statistical difference between CT and EUS (P=0.495) with regard to tumor identification. After clinical and radiological evaluation (ultrasound + CT), all the patients were deemed to be in an adequate condition to undergo surgery with a possible resection of the lesion. Table 3 shows the accuracy of tumor detection using ultrasound, CT and EUS, in relation to tumor size.
Forty-five patients (86.5%) underwent surgery; seven patients (13.5%) were followed up for a mean period of 22 months (range: 4 to 34 months) because four had presented positive results from EUS-FNA for a hepatic metastasis, two had presented lymphatic nodules in the celiac trunk and one had presented lymphatic nodules in the cervical region. All these patients had a mass in the pancreas head which was not identified on CT.
Twenty (44.4%) patients underwent exploratory laparotomy; 12 (26.7%) underwent bypass surgery; 9 (20.0%) underwent a Whipple procedure; two (4.4%) underwent subtotal pancreatectomy and two (4.4%) underwent a distal pancreatectomy. Resection of the pancreatic carcinoma was possible in 13 cases (28.9%): total resection in ten (22.2%) and partial resection in three (6.7%).
TNM Surgical Classification
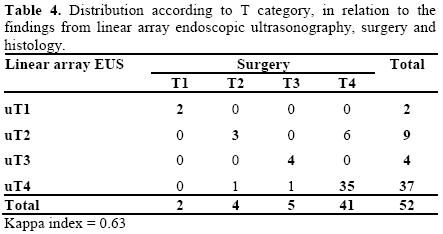
Six patients (11.5%) in our series had a tumor which was confined to the pancreatic gland (T1 and T2). Five (9.6%) were classified as T3 and presented locally advanced disease, including invasion of the peripancreatic tissues, common bile duct and duodenum. Forty-one patients (78.8%) had some type of involvement of the large vessels and were classified as T4 (Tables 4 and 5).
General Results from EUS
TN category
EUS erred in the T classification of eight patients (15.4%). Six cases were underestimated (11.5%) and two were overestimated (3.8%). In the other 44 (84.7%), there was histological and surgical confirmation of the classification from EUS (Table 4), with a kappa index of 0.63 (Figure 1).
Figure 1. a. Linear array endoscopic ultrasound image of a pancreatic carcinoma (uT4). Note the lack of clear limits between the tumor and the splenomesenteric confluence, with a thrombus in the lumen. b. Image of a hypoechoic and heterogenous tumor with poorly defined limits, invading the superior mesenteric vein.
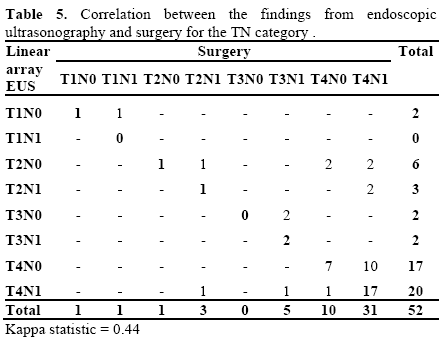
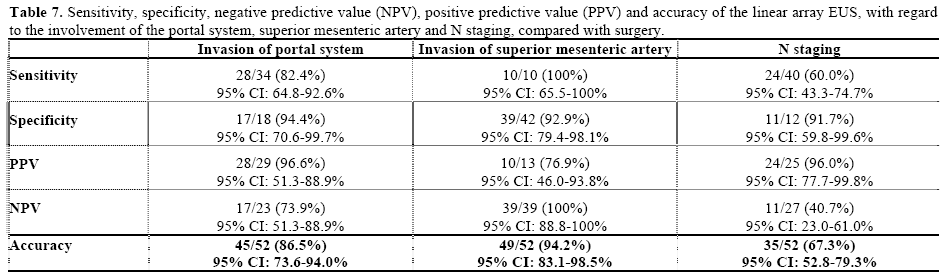
Regarding the N classification, EUS failed in 17 patients (32.6%). In the remaining 35 (67.4%), the classification proposed by EUS was confirmed by surgery and/or histology (Tables 5 and 6). Table 7 presents the results from EUS compared with surgery, with regard to evaluation of the N category, in terms of the sensitivity, specificity, PPV, NPV and accuracy (Figure 2).
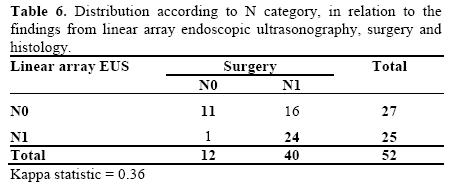
In the joint TN classification, EUS failed in 23 patients (44.2%). Three cases were overestimated (5.8%) and 20 were underestimated (38.4%). In the other 29 patients (55.8%), there was surgical and histological confirmation (Table 5), with a kappa index of 0.44.
Invasion of Large Vessels
Identification of Invasion of the Portal System
In 34 patients (65.3%), invasion of the portal system (Figure 3) was diagnosed during surgery, and was confirmed by EUS in 28/34 patients (82.4%). In 17 cases (94.4%) without invasion of the portal system, there was concordance between EUS and surgery. The statistical results are reported in Table 7.
Identification of the Invasion of the Superior Mesenteric Artery
We identified the superior mesenteric artery in 47 patients (90.4%). We were unable to find it in three patients (5.7%) with tumors located in the pancreas body and in two patients (3.8%) with tumors at the pancreatic head. The surgical findings revealed invasion in 10 patients (19.2%). Table 7 shows the statistical results from this comparison.
EUS-FNA was performed on all patients. In one patient, it was not possible to insert the needle into the mass, with breakage of the work channel of the apparatus. One hundred and twenty punctures were made, with a mean of 2.3 punctures per lesion (minimum of one and maximum of five punctures). Material for examination was obtained from 51 patients (98.1%), cytological material from 50 and fragments from 43 patients. In three cases (5.8%), the material obtained was considered insufficient for analysis. Among the 52 patients who underwent EUS-FNA, the diagnosis was correct in 42 of them, resulting in a sensitivity of 80.8% (95% CI: 64.9-89.9%). Excluding the 3 cases with insufficient material and one case of puncture failure, the sensitivity of EUS-FNA reached 87.5% (42/48 95% CI: 73.0-95.4%). Separate analysis of the sensitivity of EUS-FNA in the cases of hepatic metastasis and lymphatic nodules showed rates of 80.0%% (4/5) and 100% (5/5), respectively.
Two patients (3.8%) who underwent FNA by means of the transgastric route presented complications: hemorrhage and mild acute pancreatitis. Both of these patients were kept under observation and treated clinically, with good results
Up to now, we have not found any studies in the literature evaluating the safety and results from linear array EUS-FNA, used alone and performed only on that occasion to identify, classify in terms of T and N, and supply histopathological assessments on patients with pancreatic carcinoma who are deemed resectable according to helical CT scans.
The importance of this study stems from several factors. It was an investigation comparing the results from EUS with surgical findings relating to patients who were believed to be potentially resectable before surgery. It showed that EUS-FNA added important data to what had already been obtained using helical CT before surgery. The surgical findings were taken to be the “gold standard” for all comparisons in a large series of patients in different stages of the disease (Table 7). All the patients examined had been histologically diagnosed as pancreatic carcinoma cases. Three years after pancreatic carcinoma had been diagnosed, less than 3% of the patients were still alive. Among cases of resectable tumors (10 to 20% at the time of diagnosis), the five-year survival rate has been found to be approximately 10%, and it is zero when metastases are present [16, 17]. These data reinforce the need for improving the early diagnosis, so that the disease can be treated rationally and patients can be selected for surgery. One discouraging factor is that the evolution of surgical techniques has not improved the survival rate, following treatment for pancreatic carcinoma, and it remains approximately 18% [18, 19], with a mortality rate ranging from 4 to 18% [5, 20].
Although previous studies have suggested that radial EUS is superior to CT, studies over recent years have observed that helical CT is more precise for T staging and equivalent to the N classification [21]. Other studies have demonstrated rates ranging from 78 to 94% for the T classification and from 64 to 82% for the N classification [22]. In our sample, EUS had 84.7%, 67.3% and 55.8% accuracy, respectively for the T, N and joint TN categories, and these figures are similar to what had been obtained in previous studies. In assessing the T category, some authors have reported 85 to 93% accuracy [23, 24], with regard to concordance between radial EUS and histological examination. However, there is a tendency towards underestimation in this evaluation [25], and this occurred in 11.5% of our sample. Despite this negative finding, our statistical analysis showed good correlation between linear array EUS and surgical findings (kappa index of 0.63).
Preoperative determination of the degree of lymphatic nodule involvement is excessively difficult, independent of the method used, even though this information is extremely important [26]. Assessment of the N category showed agreement in 67.3% (95% CI: 52.8-79.3%) and a PPV of 96.0% (95% CI: 77.7- 99.8%). These results were similar to findings in the literature [10, 27]. The sensitivity and specificity of radial EUS for determining the degree of lymphatic nodule involvement is the same as CT [23, 28] and is sometimes greater than CT [25, 29].
In evaluating the TN category, our sample demonstrated correlation between linear array EUS and surgery in 55.8% of the patients. This result was inferior to another study in the literature which presented overall accuracy of 83.3% [30]. Advances in obtaining dual-phase images seem to have diminished the differences between EUS and helical CT regarding tumor detection. We saw this in analyzing the data from the present study: helical CT showed normal results for two patients in whom abdominal ultrasound revealed the presence of a nodule in the pancreatic gland. However, EUS has presented better results with regard to identifying pancreatic masses smaller than 3.0 cm in diameter [31], as well as more easily obtaining biopsy fragments and staging these small tumors [32]. Based on the sensitivity and PPV results relating to identification of tumor invasion of the portal system from EUS, it can be inferred that this method is safe and provides good diagnostic precision, particularly for tumors smaller than 3 cm [32]. In our sample, the specificity of this method regarding tumor invasion of the portal system reached 94.4%. This value is similar to results in the literature, taking the parameter for defining invasion to be either the absence of any clear hyperechoic interface between the vessel and the tumor [33] or the presence of vegetations inside the portal system [34].
The limitation of EUS in relation to evaluating the involvement of vascular structures with pancreatic carcinoma was described by Aslanian et al., who found a sensitivity of 63%, a specificity of 64%, a PPV of 43% and an NPV of 80%. In that study, as in ours, loss of the hyperechoic interface between the tumor and the vessel was taken to indicate vascular involvement. Approximately 29% of the cases presented adherence of the tumor to the vessel wall and there was no histological confirmation of invasion in any of them [35]. In the present series, the sensitivity relating to tumor involvement in the portal system was 82.4%, specificity was 94.4%, PPV was 96.6%, NPV was 73.9% and accuracy was 86.5%. A PPV of 96.6% demonstrates the elevated efficacy of this examination for precisely defining the vascular involvement with the tumor in situations in which helical CT presents doubts or determines that the tumor is resectable.
In a prospective blind study involving 62 patients, the surgical results were compared with the findings from radial EUS, helical CT and magnetic resonance imaging (MRI). The results from helical CT were superior to those from MRI and EUS, in relation to T assessment (74% vs. 62% and 68% precision, respectively). For N staging, the reported precision was 65%, 62% and 61% for radial EUS, helical CT and MRI, respectively. The precision of radial EUS for analyzing vascular invasion was 76% while it was 83% for helical CT and 74% for MRI [11]. In the present series, the results from using linear array EUS alone were better than the findings from the preceding study. The success rates for T and N were 84.6% and 67.3%, respectively and the sensitivity for vascular invasion assessment was 82.4%.
Midwinter et al. showed that there was no statistically significant difference between CT and radial EUS with regard to evaluation of tumor involvement in the portal system and in lymphatic nodules. They also demonstrated that CT was better than EUS for evaluating the involvement of the superior mesenteric artery (P<0.05) and that the accuracy of EUS for identifying pancreatic carcinoma was higher than that of CT (95.8% versus 79.2%) [28]. It should be emphasized that the evaluation of tumor involvement in the superior mesenteric artery in our series showed a PPV of 76.9% which was much lower than the results from the portal system assessment. Nonetheless, in some cases, EUS may determine portal system involvement with reasonable precision, and it should be used for this purpose, particularly in cases with tumors smaller than 3.0 cm, in which the results are much better [32]. Linear array EUS presented a specificity of 92.9% (95% CI: 79.4-98.1%) for the diagnosis of invasion of the superior mesenteric artery by the tumor, thus conferring reasonable diagnostic precision when there is no tumor involvement. The factors likely to be responsible for this precision are the distance between the transducer and the superior mesenteric artery and the ability of obtaining images without distortions, from tumors up to 4.0 cm in size. Soriano et al. suggested that radial EUS should only be performed on patients whose helical CT was inconclusive or indicated the possibility that the disease was resectable. With this approach, the classification was correct for 87% of the patients. These findings corroborate ours in which it was found that 84.7% of the cases of locoregional invasion presented potentially resectable disease. Thus, the study by Soriano et al. and ours confirm the hypothesis that helical CT used as the first imaging method should be followed sequentially by EUS-FNA as a confirmation test for potentially resectable tumors, thereby improving this preoperative strategy with regard to diagnostic efficacy and cost [11].
Hence, linear array EUS precisely defines the size of the tumor, its location and any vascular or lymphatic nodule involvement. It also provides fragments for anatomopathological study [36, 37]. Its diagnostic precision for pancreatic carcinoma cases has now been well established. The accuracy of EUS-FNA is 88%, sensitivity 86%, specificity 94%, PPV 100% and NPV 86% [37, 38]. EUS does not precisely differentiate between malignant and benign lesions [39]. EUS-FNA provides cell and tissue samples from tumors and from abdominal and/or mediastinal lymphatic nodules, and it has been used in attempts to increase the diagnostic precision regarding pancreatic lesions [30, 37]. This was proven in our series, in which the results from EUS-FNA contraindicated the surgical procedure in seven patients (13.5%) because they presented hepatic metastases or a metastatic lymphatic nodule which were not identified by helical CT but were confirmed by EUS-FNA.
Several complications have been described following EUS-FNA. Prominent among these are bleeding, perforation, pancreatitis, abdominal pain, infection and sedation-related complications. However, in experienced hands, the complication rate following EUS-FNA on solid pancreatic masses is similar to that found in upper digestive endoscopy [40]. In a prospective study on 355 patients, the general complication rate reported was 2.54% with 0% mortality. The most common complications were acute pancreatitis, abdominal pain, fever and problems relating to sedation. In our series, the complication rate was 3.8%: one case each of bleeding and acute pancreatitis, which were both treated clinically. The risk of acute pancreatitis following EUS-FNA in a retrospective study was found to be 0.29% and, in a prospective study, it was 0.85%, in contrast to 3% in cases of percutaneous biopsy [41, 42, 43, 44]. In the present series, the risk of an episode of acute pancreatitis following EUS-FNA was 1.9%. Other important limitations include the operator’s experience, the need for adequate sedation, the duration of the process (in comparison with CT), the need for an experienced gastrointestinal pathologist and the limited availability of EUS within the zone.
It seems reasonable to indicate EUS-FNA for patients with a suspected pancreatic carcinoma, despite previously performing helical CT, because of its high accuracy of tumor identification, along with the wide range of information provided. Histological diagnosis of the tumor and of metastases (lymphatic nodule and hepatic metastases), staging assessments (T and N), contraindication of unnecessary laparotomy and pain treatment for such patients, all rationally in a single procedure, form part of the many activities which can be accomplished by means of linear array EUS.
This study shows the role of EUS-FNA for TN staging in patients with proven ductal pancreatic carcinoma and the role of this technique in the preoperative staging of patients with ductal pancreatic carcinoma where helical CT showed evidence of resectability of the tumor.
This research did not receive any financial support. The authors have no potential conflicts of interest