Mini Review - (2016) Volume 2, Issue 1
Abhilasha Tiwari1,2*, Guy Moeneclaey1,2, Graham Jenkin1,3 and Mark A Kirkland2,4
The Ritchie Centre, Hudson Institute of Medical Research, Monash University, Clayton, Australia
Cytomatrix, Melbourne, Victoria, Australia
Department of Obstetrics and Gynaecology, Monash University, The Ritchie Centre, Hudson Institute of Medical Research, Monash University, Clayton, Australia
Geelong Technology Precinct, Deakin University, Geelong, Victoria, Australia
Corresponding Author:
Abhilasha Tiwari
The Ritchie Centre, Hudson Institute of Medical Research
Monash University, Clayton
Australia
Email: abhilasha.tiwari@gmail.com
Received date: Jan 05, 2016; Accepted date: Feb 23, 2016; Published date: Feb 26, 2016
Hematopoietic stem cells (HSCs) are responsible for maintenance and production of functional blood cells. Each day the human body produces billions of new white blood cells, red blood cells, and platelets to replace blood cells lost to normal cell turnover processes as well as to illness or trauma. The highly orchestrated process of blood cell production and homeostasis is termed hematopoiesis. HSCs circulate around the blood during fetal stage and reside in the bone marrow of adults.
Keywords
Stem cells; Trauma; Hematopoiesis; Umbilical cord; Blood; Transplantation
Mini Review
Hematopoietic stem cells (HSCs) are responsible for maintenance and production of functional blood cells. Each day the human body produces billions of new white blood cells, red blood cells, and platelets to replace blood cells lost to normal cell turnover processes as well as to illness or trauma [1-3]. The highly orchestrated process of blood cell production and homeostasis is termed hematopoiesis. HSCs circulate around the blood during fetal stage and reside in the bone marrow of adults [4].
For clinical purposes, HSCs can be obtained from mobilized peripheral blood, bone marrow and umbilical cord blood (UCB). UCB is a valuable source of HSCs and effective alternative for bone marrow transplantation. The first hematopoietic stem cells transplantation (HSCT) of cord blood was carried out in 1988 with more than 35,000 transplantations till date [5,6]. UCB stem cells are incredibly easy to collect and cryogenically freeze as compared to the collection of bone marrow that requires an invasive procedure. UCB stem cells are more immature and less immunogenic than stem cells taken from an adult, which gives them better regenerative abilities with lower incidence and severity of “Graft versus Host disease” (GvHD) [7,8]. Provided that there is enough stem cells available in the graft, transplantation using UCB allows for a greater HLA-disparity between the donor and recipient as compared to that from bone marrow (BM) and mobilized peripheral blood (mPB) [9]. As a result cord blood banks have been established around the world to provide a source of UCB for transplant [10,11] (Figure 1).
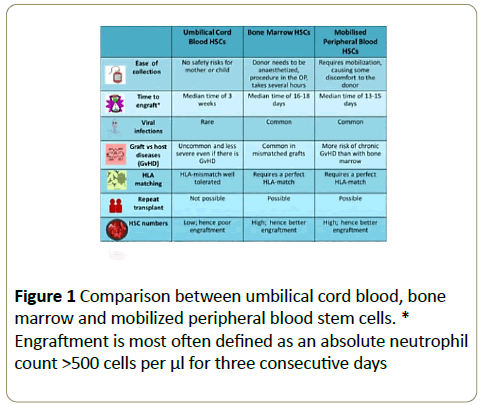
Figure 1: Comparison between umbilical cord blood, bone marrow and mobilized peripheral blood stem cells. * Engraftment is most often defined as an absolute neutrophil count >500 cells per μl for three consecutive days
However, the transplantation of UCB cells has two major disadvantages: (i) the low number of HSPCs in an average UCB unit (average volume of 75ml blood per umbilical cord yields approximately 2.2 106 CD34+ cells) limits its application to children only (ii) neutrophil and platelets in UCB-transplanted patients need a longer recovery time than in BM or mobilized PB following transplantation [8,12]. Hence, ability to expand human HSPCs ex-vivo is clearly an enormous boost to all current and future medical uses [13-15].
To date, UCBT has been applied in over 90 indications, however, most of them are blood related disease [16,17]. It is now currently estimated that 1 in 3 people might benefit from therapy using cord blood stem cells in their lifetime [18,19] (Figure 2).
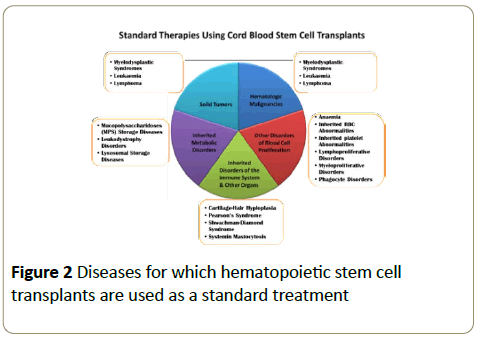
Figure 2: Diseases for which hematopoietic stem cell transplants are used as a standard treatment
There is an expanding range of research and clinical trials evaluating how cord blood stem cells may contribute to new therapies for a broad number of conditions. New fields of regenerative medicine are investigating cord blood as a potential source of reparative stem cells for conditions including; cerebral palsy, type 1 diabetes, autism, hearing loss and stroke. Several clinical trials have been initiated to investigate the application of autologous or allogeneic cord blood stem cells for treatment of a number of neurological, autoimmune and cardiovascular disorders [17-21].
Some current and future trails that are possible using these cells are listed below:
• Autism is a spectrum disorder affecting about 1 in 68 children and known to be caused by certain gene factors involved in brain development and early life environment. Using autologous cord blood, a study at the Sutter Neuroscience Institute California (NCT01638819) aims to potentially reverse the effects of this disease and demonstrate improved behavior and learning, where it is not attributable to other factors such as genetic disorders, head injury or prematurity [22].
• Cerebral injury reduces or limits supply of blood and thus oxygen to the brain, resulting in the death of brain tissue. There are currently seven clinical trials, investigating the use of cord blood as a treatment for cerebral injury in newborn or young children [4,23].
• There are two primary causes of stroke-bleeding on the brain and a clot in the artery supplying blood to the brain. Both result in the loss of oxygen to brain tissue. Much work has been done in rats as a model of the disease to improved neurological function into the affected brain. Study demonstrates that direct injection of cultured cordderived mesenchymal stem cells (MSCs) to the lesion can be effective [24-26]. Another study is being conducted by researchers at the China Medical University Hospital in Taiwan. The purpose of the study is to determine the safety and effectiveness of brain transplants of CD34+ stem cells obtained from umbilical cord blood (NCT01438593).
• Cerebral palsy that affects 1 in every 323 children is caused by a brain injury or lack of oxygen in the brain before birth or during the first few years of life, can impair movement, learning, hearing, vision, and cognitive skills. There is currently six clinical trials listed using cord blood for treatment of cerebral palsy. Two of the most prestigious research institutes – Duke University and Georgia Health Sciences Institute-are working on clinical trials in this area (NCT01072370 and NCT01147653) [23,27]. Another study from Korea has already completed the trail and demonstrated that UCB treatment ameliorated motor and cognitive dysfunction in children with cerebral palsy undergoing active rehabilitation [28].
• Multiple sclerosis (MS) is an autoimmune response that destroys the myelin sheath that protects the nerves in the brain and spinal cord leading to severe disability and early death. A study is being conducted by the Stem Cell Institute in Panama is to assess the safety and effectiveness of donor (allogeneic) umbilical cord MSCs administered to patients with MS (NCT02034188). Another phase IIa study was performed on a small patient cohort and showed some improvement in vision, but limited impact on disease progression, measured by disability worsening [29,30].
• Huntington’s disease is a progressive brain disorder resulting in the slow loss of brain cells. A stem cell-based treatment for Huntington’s disease is many years away, yet current research aims to use stem cell technologies to understand and work on gene therapy and symptomatic treatment. UCB-derived stem cells can play an important role in this as they are easier to transduce as compared to mPB and BM [31,32].
• Parkinson’s disease is caused by a lack of dopamine due to nerve cell death in the brain. At present no treatments are available for this disease. It is hoped that the cell line work in modelling this disease will lead to therapies in the future [33,34].
• Rheumatism is caused by the immune system attacking the lining of the joints, which causes pain, inflammation, swelling, permanent joint damage and deformity. There are currently 15 registered trials using stem cells to treat rheumatoid arthritis. NCT01547091 is looking to use umbilical cord-derived MSCs to treat this [35].
• Lupus is a chronic disorder of the immune system that results in too many antibodies being produced. This causes inflammation that may affect multiple organs of the body. There is one current clinical trial (NCT00278590) recruiting patients to look at the use of stem cell transplant [36].
• Graft versus host disease (GVHD) occurs when a transplant of human tissue such as blood or organs from a donor to a recipient is attacked by the recipient’s immune system. A phase II study at the Karolinska Institute is now being followed up in a European randomised study [37,38].
• Myocardial infarction is usually caused by a blood clot that prevents flow to part of the heart muscle. The lack of oxygen causes tissue death, which leads to scar tissue formation that ultimately weakens and reduces heart functionality. There have been 13 trials to date using stem cells to treat patients by repairing the damage to the muscle [39,40].
• Sickle cell disease is a form of anaemia resulting from a genetic abnormality in the haemoglobin-producing genes, and is usually inherited. It is known that transplantation of HSCs from UCB can treat sickle cell disease successfully. Clinical trial NCT00029380 demonstrates this, with results to be confirmed after follow up. A trial with expanded cells is being planned [41].
• Type 1 diabetes is an autoimmune disease that causes the beta cells of the pancreas to be destroyed. This results in insufficient insulin being produced, and therefore uncontrolled sugar levels in the blood. Currently, the clinical trials registry notes 47 trials investigating treatments of type 1 diabetes using cord blood stem cells [42].
• Umbilical cord stem cells are also a good choice for gene therapy because they are well characterised and the clinical transplantation protocols are well established. UCB stem cells can be used to correct genetic deficiencies at birth with possibly higher efficiency of gene transfer as compared to adult stem cell transplants [43].
Since the first reports by the French team with Prof. Gluckman [44] cord blood has been recognized as a valid alternative for stem cell transplantation for a variety of indications including malignant and non-malignant diseases. UCB cells are currently being investigated for use in gene therapy protocols, for treatment of autoimmune diseases but also in tissue regeneration and engineering applications e.g. organ regeneration (liver, pancreatic, neural tissues and more). Increasing the number of HSC from the UCB unit will be crucial to the success of transplantation in adult settings where a larger number of stem cells are required in order to minimize the risk for the recipient, to shorten the time to engraftment and to lower the transplantation-related morbidity and mortality. Several researchers are currently investigating how to reliably and reproducibly increase the stem cell pool that can be obtained from a cord blood unit, and how to maintain their stemness [7,45-47]. Larger clinical trials will be necessary to better understand and confirm the potential of these precious cells and to confirm their applicability for treatment of a vast range of indications.