- (2011) Volume 12, Issue 4
María del Carmen Gómez Mateo1, Elena Muñoz Forner2, Luis Sabater Ortí2,3, Antonio Ferrández Izquierdo1,3
Departments of 1Pathology and 2Surgery, Clinical Hospital, 3University of Valencia, Valencia, Spain
Received May 6th, 2011 - Accepted June 3rd, 2011
Context Foregut cystic malformations are common lesions in the mediastinum but are rarely found in subdiaphragmatic locations. Only a few cases have been described within the pancreas where they can easily be misdiagnosed as cystic neoplasms. Case report We herein present the case of a 37-year-old female with acute cholangitis in whom a diagnostic work-up revealed a 1 cm solid-cystic heterogeneous lesion located at the head of the pancreas. The patient underwent a pancreaticoduodenectomy. Pathological evaluation demonstrated a cystic cavity lined by pseudostratified tall columnar ciliated epithelium with goblet cells, but lacking cartilage or smooth muscle bundles. Thus, the final diagnosis of the lesion was a ciliated foregut cyst of the pancreas. Conclusions A review of the cases published regarding these lesions shows great variability in the taxonomy and a lack of accuracy in the definitions of each different subtype. An easy to use algorithm for the diagnosis of foregut cystic malformations subtypes, based on epithelial lining and wall features, is presented.
Bronchogenic Cyst; Congenital Abnormalities; Mediastinal Cyst; Pancreas; Pancreatic Cyst
The most common cystic lesions of the pancreas are pseudocysts. Malignant neoplastic cysts must be differentiated from benign cystic and pseudocyst lesions, such as congenital, parasitic, retention or endometrial cysts [1].
True cysts of the pancreas are characterized by an epithelial lining and are subdivided into acquired and congenital or developmental cysts [2]. Foregut cystic malformations are a type of true congenital cyst, quite common in the mediastinum, uncommon in the hepatobiliary system with approximately one hundred cases described [3] and extremely rare in or around the pancreas with only 14 cases previously reported [1, 4].
Foregut cystic malformations can be lined by ciliated columnar or pseudostratified epithelium and goblet cells. When they also contain respiratory glands or cartilage, they are defined as bronchogenic cysts whereas the presence of two smooth-muscle layers indicates an esophageal or gastroenteric differentiation. A cyst with ciliated epithelium, with no other additional defining features, is properly referred to as a “ciliated foregut cyst” [1, 5].
The aim of this paper is threefold: a) to present a rare case of foregut cystic malformation of the pancreas with illustrative and clarifying iconography; b) to point out the confusion in the terminology used; an extensive review of the previously reported cases of foregut cystic malformations of the pancreas shows a high index of misclassification according to recent diagnosis criteria [6] and c) to offer a simple diagnostic algorithm of the different foregut cystic malformations, according to the characteristics of the epithelial lining and wall features.
A 37-year-old female presented at the emergency department after two days of abdominal pain, fever and nausea. She had no prior medical history or weight loss. On physical examination, she was jaundiced, and palpation of the right upper quadrant was clearly painful without rebound tenderness. Laboratory tests revealed an elevated white blood cell count (11x109/L; reference range: 4.0-10.8x109/L) and bilirubin of 3.1 mg/dL (reference range: 0.10-1.00 mg/dL). Other values of blood chemistry and coagulation were normal. An abdominal ultrasound showed cholelithiasis and dilatation of the common bile duct (11 mm) with no clear evidence as to the cause of the obstruction. The clinical picture was diagnosed as acute cholangitis and was treated with intravenous antibiotic therapy for 5 days. An additional diagnostic work-up included abdominal CT and MRI which revealed a 1 cm solid-cystic lesion located at the head of the pancreas, between the main pancreatic duct and the distal common bile duct. An endoscopic ultrasound confirmed a solid-cystic tumor with a diameter of 9 mm in the pancreatic head with no signs of vascular infiltration but with partial compression of the biliary duct. Serum tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19- 9), were within normal values. Based on extensive diagnostic procedures which could not rule out malignancy and obstructive jaundice and which seemed to be secondary to the bile duct compression by the cyst, we proceeded with surgical resection. A pancreaticoduodenectomy (Whipple’s procedure) was performed; the postoperative course was uneventful and the patient was discharged 10 days postoperatively.
Macroscopic Evaluation
Macroscopic study was performed according to the new standards for the management and handling of pancreatic specimens [7, 8]. The sample was measured, the retroperitoneal margins were inked in using three different colors and it was sectioned into 0.5 cm slices perpendicular to the duodenal axis. Gross examination of the resection specimen revealed a 0.9 cm unilocular cyst. The lining of the cyst was smooth. Serial cut sections of the specimen revealed the cyst to be near the duodenum and adjacent to the common bile duct (Figure 1).
Histology
Microscopic examination revealed a cystic cavity lined by pseudostratified ciliated epithelium with interspersed goblet cells, overlying loose connective tissue. This was accompanied by a slight inflammatory infiltrate, but no cartilage or smooth muscle bundles were found. The pancreatic tissue surrounding the cyst showed neither atrophy nor inflammation (Figure 2). Immunohistochemical analysis of the formalin-fixed paraffin-embedded sections demonstrated focal cytoplasmic staining in the cyst epithelium for CEA (1:100 dilution). Staining was also positive for cytokeratin 7 (CK7) (1:100 dilution). Thyroid transcription factor 1 (TTF-1) (prediluted), cytokeratin 20 (CK20) (1:100 dilution) and homeobox protein CDX2 (1:100 dilution) were negative. In addition, rare chromogranin (1:100 dilution) and synaptophysin positive cells (1:100 dilution) were found interspersed within the epithelial layer (Figure 3). All stains were from Dako (Copenhagen, Denmark).
Figure 2. a. Panoramic view of the cystic lesion, with compression of the bile duct (arrow). (H&E, 100x). b. The cystic lining is composed of a thin layer of epithelium (arrowhead) covering the loose connective tissue without smooth muscle bundles or cartilage. Normal pancreatic surrounding (star). (H&E, 400x) c. A detail of pseudostratified ciliated epithelium with interspersed goblet cells (arrows) (H&E, 630x).
Figure 3. Different immunohistochemical results of the cystic lining (400x). Positive staining with cytokeratin 7 (CK7; a.) and CEA (b.). Immunostaining of interspersed cells with chromogranin (c.) and synaptophysin (d.). Negative staining with thyroid transcription factor 1 (TTF-1; e.), homeobox protein CDX2 (f.) and cytokeratin 20 (CK20; g.).
Foregut cystic malformations are frequent in the mediastinum, where they account for approximately 20% of all benign mediastinal masses [9]. However, they occur very rarely in the pancreatic and hepatobiliary system and very few cases have been described [3].
Foregut cystic malformations arise in relation to the development of the esophagus and tracheobronchial tree and can be classified into different types, depending on the epithelial lining and the features of the wall. Historically, a variety of terms have been used to refer to cysts of foregut origin, including gastric cysts, epithelial cysts, esophageal cysts, enteric cysts, bronchogenic cysts, foregut cysts, esophageal duplication cysts, cysts of foregut origins, bronchopulmonary foregut cystic malformations, cyst with intestinal epithelium, biliary cysts, congenital duplication cysts, cystic duplications, and enterocystomas [6]. However, these terms are not always interchangeable. This diverse terminology may be due to the fact that foregut cystic malformations can also be present in different locations of the abdomen where cases are not as frequent as in the mediastinum, thus involving different medical specialties (embryology, anatomy, pathology, thoracic surgery, pediatric surgery and general abdominal surgery). Therefore, diverse classifications with overlapping groups have originated as shown in Figure 4, adding confusion to the literature available [6].
Figure 4. Scheme showing the overlapping terms of foregut cystic
malformations.
BC: bronchogenic cyst; CFC: ciliated foregut cyst; DC: duplication
cysts; ENC: enterogenous or enteric cyst; ESC: esophageal cyst (not
ciliated); ESCC: esophageal cyst (ciliated epithelial lining); FC:
foregut cysts (ciliated); FCM: foregut cystic malformations; GC:
gastric cyst; GEC: gastroenteric cyst
In a comprehensive and simplified manner, this type of cystic malformation can be classified as a foregut cystic malformation in general. Cases with a double layer of smooth muscle in their wall are classified, depending on the epithelial lining. When the epithelium is squamous, columnar (ciliated or not) or a mixture of these, the appropriate term is esophageal cyst. When the lining simulates the epithelium of either the gastric or the normal intestine, they are defined as gastric and enteric or enterogenous cysts. Occasionally, combined forms may be found and are called gastroenteric cysts [10]. Bronchopulmonary or bronchogenic cysts are usually lined by ciliated columnar epithelium and may contain cartilage, smooth muscle, bronchial glands and nerves in the wall. When a cyst with ciliated columnar epithelium has no additional defining features, it is properly referred to as a ciliated foregut cyst [1, 5] (Figure 5).
Foregut cystic malformations arising in relation to the development of the liver and pancreas are hypothesized to occur from a detached outpouching and sequestering of the primitive foregut within the liver and pancreatic tissue during embryological development [1]. By the 4th week of fetal development, a primitive diverticulum arises from the ventral part of the cranial foregut. A septum then divides this primitive foregut into a dorsal esophagus and a ventral respiratory laryngotracheal tube. The esophagus, stomach, duodenum, liver, gallbladder and pancreas derive from the dorsal tube while the ventral part will form the bronchopulmonary system. The respiratory tube is lined by pseudostratified ciliated columnar epithelium and the dorsal tube acquires squamous or columnar (gastric or intestinal type) epithelium. Foregut cysts arise from an abnormal budding of the primitive foregut. When the attachment persists, the cyst is usually found near or associated with the tracheobronchial tree or the esophagus. If a complete separation occurs, the cyst may migrate to other unusual locations. A retroperitoneal location is extremely rare, and the exact mechanism of its migration is still unknown [11].
Histologically, the walls of ciliated foregut cysts are lined by a layer of pseudostratified ciliated epithelium interspersed with goblet cells (also called respiratorylike epithelium). Lymphocytes may be present in the cystic wall. The epithelial cells have been reported to stain for CK7, CEA [3, 12] and CA 19-9 [1, 4] and are non-immunoreactive for CK20 and homeobox protein CDX2 [1, 4, 12]. Staining for thyroid transcription factor 1 (TTF-1) has been reported to be positive in one case [12] and negative in two cases [1, 4] as was found in this case. In addition, scattered neuroendocrine cells within the epithelial lining can be demonstrated with chromogranin and synaptophysin [1, 3].
Ciliated foregut cysts contain material ranging from clear serous fluid to milky white or brown viscid, mucoid material with abundant lipid and protein content [2, 13, 14]. Reports in the literature describe the presence of increased concentrations of carcinoembryonic and CA 125 antigens [1, 14, 15], typical features for a mucinous cystic neoplasm of the pancreas. Even though they are considered benign, four cases have been described which have progressed to malignancy in ciliated hepatic foregut cysts [16, 17, 18, 19] and one case of metastatic adenocarcinoma in an esophageal cyst [20]. Therefore, even in cases with an established diagnosis of foregut cystic malformation, observation alone should not be recommended.
According to recent reviews [1, 4], fourteen cases of “ciliated foregut cyst” arising in the pancreas have been reported in the English literature since the first case described in 1969 by Lyon [21]. All such cases have been summarized in Table 1 in order to show the specific characteristics of each cyst and the great confusion of the terminology used.
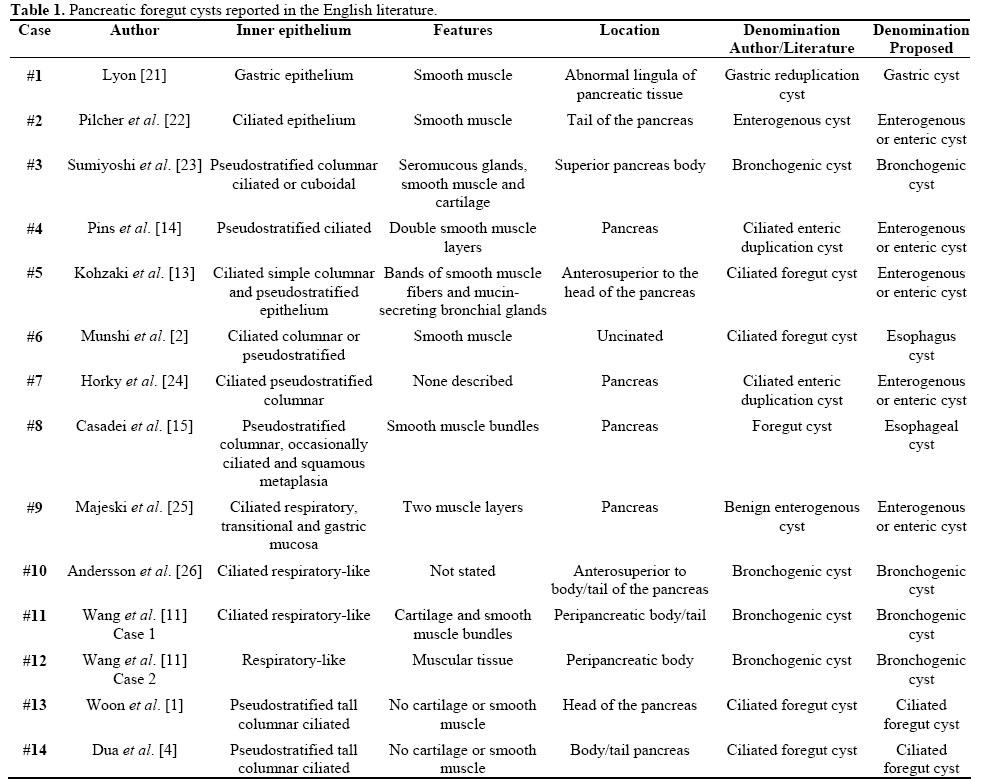
Following the classification of foregut cysts appearing in Ackerman’s Surgical Pathology [10] and in agreement with a recent review of terms by Sharma et al. [6], the cases were divided into 4 bronchogenic cysts (Cases #3, #10, #11 and #12), 8 esophagicgastric- enteric cysts (Cases #1, #2, #4, #5, #6, #7, #8, and #9), and only 2 cases (Cases #13 and #14) appropriately referred to as ciliated foregut cysts. According to the definition of Harvell et al. [5] and Sharma et al. [6], when the wall of the cyst is lined by ciliated columnar epithelium and has no other additional defining features, such as cartilage or smooth muscle, it should be called a ciliated foregut cyst. Our case corresponds to the latter term, thus making it the third real case properly described as a ciliated foregut cyst of the pancreas.
The authors have no potential conflict of interest
We would like to thank Ms. Landy Menzies for her kind assistance in preparing the English version of the manuscript