- (2005) Volume 6, Issue 4
Ake Andren-Sandberg1, Philip D Hardt2
1Department of Gastrointestinal Surgery, Sntralsjukehuset i Rogaland, Bergen University. Stavanger, Norway. 2Third Medical Department, Giessen University Hospital. Giessen, Germany
The ‘Giessen International Workshop on Interactions of Exocrine and Endocrine Pancreatic Diseases’ was held on March 18- 19, 2005 at the Castle of Rauischolzhausen, Giessen University, Germany. About 50 international clinicians and researchers attended the workshop. It was structured into three sessions: A: Pancreatic Autoimmunity - Interaction Between Exocrine and Endocrine Tissue; B: Diabetes Mellitus - Possible Implications of Exocrine Pancreatic Insufficiency; C: Chronic Pancreatitis - Update on Prevalence, Understanding and Pathophysiological Concepts. Several new aspects of pancreatic diseases were discussed, including new classifications of pancreatitis, new insights into prevalence, pathophysiology and new therapeutical considerations. The meeting resulted in more cooperation and a number of new concepts for clinical study which will provide data for future developments.
Autoimmunity; Congresses; Diabetes Mellitus; Islets of Langerhans; Pancreas, Exocrine; Pancreatitis
ANA: anti-nuclear antibody, ASMA: anti-smooth muscle antibody; CA II: carbonic anhydrase type II; ESTHER: Epidemiologische Studie zu Chancen der Verhütung, Früherkennung und optimierten THerapie chronischer ERkrankungen; GFAP: glial fibrillary acidic protein; GLP-1 ICA: islet cell antibodies; JPS: Japan Pancreas Society; MHC: major histocompatibility complex; PDGF platelet-derived growth factor; PP: pancreatic polypeptide; PRSS1: protease serine 1 (trypsin I precursor); PSC: pancreatic stellate cell; PSP: pancreatic stone protein; PYY peptide YY; SOD: sphincter of Oddi stenosis
The Department of Endocrinology and Gastroenterology of the Justus-Liebig- University in Giessen, Germany has for many years, been involved in the management of patients with both endocrine and exocrine pancreatic insufficiency. It has been observed that, when caring for patients with different clinical forms of diabetes mellitus, many of them also show morphological and functional changes of the exocrine pancreas. In a number of clinical trials, it was learnt that:
1. exocrine insufficiency is very frequent, not only in type 1 but also in type 2 diabetes mellitus;
2. a relevant percentage of these patients exhibit quantitative fat maldigestion;
3. quite a few of them fit the diagnostic criteria of chronic pancreatitis, i.e. that diabetes secondary to pancreatitis might be more common than believed so far;
4. chronic pancreatitis is much more frequent than previously believed and appears to be associated with chronic obstruction and gallstone disease;
This was the basis for an international workshop in Giessen on “Interaction of Exocrine and Endocrine Pancreatic Diseases” arranged by Philip D Hardt, Hans Ulrich Klör and Reinhard G Bretzel and attended by about 50 researchers and clinicians during one and an half days in a perfect setting.
The meeting was held on one of the first days of spring in a castle in Hessen (Figure 1). The castle was first built in the 8th century and got its name from Lord Rau in 1369. The present castle is from the 1870s and is reminiscent of an elegant English manor house; nowadays, it is used by the University of Giessen mainly as a site for studies in agriculture.
Hans Ulrich Klör (Giessen, Germany) introduced the symposium by asking some important questions.
How Common is Chronic Pancreatitis?
Is it just a rare disease in middle-aged, thin male drug addicts (alcohol, nicotine), easily diagnosed by tattoos on their bodies or is it rather a widespread disease among middle age to elderly people in Western and 'westernized' countries, not easily diagnosed in clinical practice? If the second statement is the correct answer, it must be asked what impact this exocrine disease has on the global epidemic of endocrine disease (i.e. diabetes mellitus). We should remember that a worldwide increase in diabetes is expected during the next 20 years. There were 151 million diabetics in the world in 2000, but in 2010 there will probably be about 221 million diabetics (i.e. an increase of 46%).
What Are the Mechanisms Linking Exocrine and Endocrine Pancreatic Disease?
Are the chronic inflammatory destructive processes due to: a) genetic abnormalities facilitating and maintaining inflammation, autoimmune aggression against both exocrine and endocrine cells, b) chronic exposure to toxic substances (e.g. alcohol or infectious agents) or c) an obstruction of secretory flow triggered by the daily dietary challenge of the 'western' lifestyle in conjunction with one or more of the factors mentioned above?
Then, if exocrine disease is an important contributor to diabetes mellitus:
How Should we Change the Approach to the Diagnosis and Treatment of Chronic Pancreatitis?
Which diagnostic tools should we use: routine function tests (e.g. stool elastase), imaging methods (e.g. MR, CT), ERCP or endosonography? Moreover, which therapeutic modalities should be used: for example, permanent elimination of obstructive pressure by 'sequential minipapillotomy' ('free flow forever'), early enzyme replacement therapy (preserving the incretin stimulus) or chronic antiinflammatory treatment?
Klör also asked for good intervention trials to evaluate therapeutic modalities for 'mild' chronic pancreatitis in order to prevent progression to islet cell failure. Another important step forward would be results from a supportive animal model.
The first lecture was given by Åke Andrén- Sandberg, (Stavanger-Bergen, Norway) on some aspects of pancreatic history. The pancreas was named by Rufus of Ephesus in 100 A.D. [1], who thought it to be an organ 'totally of flesh', i.e. not containing bones or cartilage. Andreas Vesalius [2], in the fifth volume of his work on human anatomy, did not recognize its excretory duct. It was not until 1642 that Johann Georg Wirsüng [3], an anatomist in Padua (Italy), described the pancreatic duct. He probably did not understand what he saw, but he sent seven copper plates around Europe (the part of the world he knew). However, at that time, no one could explain why there should be a duct there. Hundreds of years later, Santorini [4] described the minor pancreatic duct (which had already been described by Frederik Ruysch in 1665) [5]. Soon afterwards, Ruggero Oddi [6], at the age of 23, rediscovered the sphincter of the bile duct. Regnier de Graf in London [7], also at an early age, described the pancreas as a secretory organ for the first time. In 1673, Johann C Brunner [8], then a student in Paris (France), performed the first pancreatectomy in a dog to determine its function; Brunner fared better than the dog.
Claude Bernard, in 1856 [9], was the first important contributor to the physiology of the organ in his experimental studies of the action of pancreatic juice in food digestion. He let a candle bathe in pancreatic juice and saw it melt away and thus understood the digestive properties of pancreatic juice. Later, the work of Valentine explained the effect of pancreatic juice on starch.
Although diabetes is a disease which has been known since ancient times, it was little more than 100 years ago (1889) that Joseph Freiherr von Mering and Oskar Minkowski [10] performed a total pancreatectomy in a dog to see if it could survive without a pancreas. Following the operation, a technician noted flies around the urine of the dog, an observation which led to the discovery that the pancreas is responsible for glucose regulation. Paul Langerhans [11], a student of Rudolf Virchow in Berlin, had earlier described the endocrine pancreas for the first time (in 1869). Langerhans, while still a medical student in Berlin, published his remarkable histologic studies of the pancreas as his inaugural dissertation, but the pancreas as being the site of the origin of diabetes mellitus not was revealed until 1881. Using the transillumination method, as Lister did in his study of inflammation, Langerhans described several types of cells and tissues in the pancreas and, by using his own original method of dye injections, he showed the acinar tissue to be separated from the islet tissues. These collections of cells were given scant notice until the French histologist Laguesse recognized them and their special anatomic features, and in 1896 called them les ilots de Langerhans [12]. Insulin was discovered in 1921 by Banting, a young surgeon, and Best, his medical student in Toronto [13], but before that both secretin and gastrin had been described. By positive and conclusive experimental surgery published in 1922, the internal secretion of the pancreas, since then named insulin, has been applied therapeutically in the treatment of diabetes. The first injection was ordered in writing by J.J.R. MacLeod [14]. Insulin was then marketed in less than two years under at least nine different brands in North and South America. The Nobel Prize was awarded to Banting and MacLeod in 1923; Banting appropriately shared his prize and credit with Best.
Looking back at history, this lecture pointed out that the physiology and the pathology of the pancreas have only been understood rather recently. It was not until the 20th century that there was a separation into endocrinology, with researchers interested in islet function, and gastroenterology, and with researchers focusing mainly on exocrine tissue. Therefore, this workshop aimed to bring researchers of the endocrine and the exocrine pancreas back together to discuss the diseases and interaction of both parts of this organ together.
The Workshop was structured into three sessions:
Session A: “Pancreatic Autoimmunity - Interaction Between Exocrine And Endocrine Tissue” was chaired by Günter Klöppel (Kiel, Germany) and Hans Ulrich Klör (Giessen, Germany);
Session B: “Diabetes Mellitus - Possible Implications of Exocrine Pancreatic Insufficiency” was moderated by Guido Adler (Ulm, Germany) and Clement W Imrie (Glasgow, United Kingdom);
Session C: “Chronic Pancreatitis - Update on Prevalence, Understanding and Pathophysiological Concepts” was presented by Phillip P Toskes (Gainesville, FL, USA) and Philip D Hardt (Giessen, Germany).
This manuscript summarizes the major aspects of the workshop arranged by main contents.
Roland H Pfützer (Mannheim, Germany) discussed the “Impact of Genetics on Chronic Pancreatitis and Autoimmunity” and started by acknowledging the work of David Whitcomb on hereditary pancreatitis. Today, there are 19 known mutations attributed to the cationic trypsinogen gene: R122H (more than 100 families), N26I (more than 50 families) and A16V (more than 10 families). In all these mutations, there is a gain of function.
The cAMP-sensitive chloride channels are present in most epithelia. A loss of function results in a thickened secretion with a loss of ability to hydrate macromolecules. In the pancreas, these channels are the driving force of bicarbonate secretion. Mutations in the CFTR gene are associated with 'idiopathic', familial and alcoholic chronic pancreatitis. The relationship between mutations of the cystic fibrosis gene and idiopathic pancreatitis was first discussed by Cohn et al. in 1998 [15] and mutations of the cystic fibrosis gene in patients with chronic pancreatitis were reported by Sharer et al. in the same year [16].
SPINK1/PSTI is an intrapancreatic intracellular trypsin inhibitor which blocks activated trypsin by entering into a specific 'pocket'. The most common mutation is N34S, but the mechanism of action is still unclear. The frequency in a general population is about 1%, i.e. almost a polymorphism. The frequency of genetic variations in patients with chronic pancreatitis of different origin is well-described in Table 1.

It was concluded that there is a strong genetic basis for pancreatitis, but, despite that, the development of chronic disease cannot be explained by mutations only. PRSS1 mutations are not limited to autosomal dominant pancreatitis (e.g. A16V) and SPINK1 and CFTR mutations play a role in almost all kinds of pancreatitis, but the heterogeneity of disease expression cannot be explained by these mutations.
It should also be remembered that there were several reports of genetic influence on pancreatitis as early as 1978. For example, according to Dani et al., AW23 and AW24 were associated with chronic calcifying pancreatitis of alcoholic origin [17], and HLA A and B antigens in chronic alcoholic pancreatitis were discussed by Gosselin et al. the same year [18] and Fauchet et al. the year after [19].
The genes of the MHC-HLA complex are partially found with a 4Mb region on 6p21.3, where there are a multitude of genes which are important to the immune function. There are three classes (Table 2).

Moreover, there are also a lot of alleles of the MHC-HLA complex (for some loci, e.g. HLA B 490 are described), and the same is seen of HLA antigens in type 1 diabetes. This means that genetic variability at the different HLA loci may determine individual susceptibility to chronic pancreatitis of various origins, but recent studies are hampered by incomplete haplotype analyses and small sample sizes (i.e. the role of associated genotypes and haplotypes remains to be determined).
Classification
There are several ways of classifying chronic pancreatitis today. Luca Frulloni (Verona, Italy) introduced the “Verona Classification for Pancreatitis”, based on the hypothesis of 'different diseases, common progression to chronic stage if the cause is not removed' (Table 3).

Frulloni stated that, in Verona, alcohol consumption was not considered an etiological factor per se in chronic pancreatitis. Instead, he and his co-workers believed that alcohol accelerates a chronic inflammation begun by other factors. That means that autoimmune pancreatitis may be more important than just what the statistics indicate as probable; many patients with 'alcohol-associated pancreatitis' may have another cause, e.g. autoimmunity.
Epidemiology
Dietrich Rothenbacher (Heidelberg, Germany) discussed the 'epidemiological aspects' of chronic pancreatitis. He was very straightforward from the start: “We don’t know the true incidence and prevalence in the population”. Today we only know figures from hospitals and then mainly from hospital referral centres where doctors probably have a special interest in the disease and attract more patients with diseases where treatment had failed in other instances. It must also be accepted that the incidence depends on the knowledge of the doctors, the interest of the doctors, and the possibility of treating the patients - but also on whether good screening possibilities exist. A good example of interest and knowledge is a study performed in Saarland, Germany. It included 55-75 yearold healthy individuals (ESTHER study [20] https://www.estherstudie.de). In a subgroup of 914 people, fecal elastase 1 measurements were taken in order to learn about the prevalence of exocrine dysfunction in a population-based sample. Elastase 1 measurements were used, because this test appears to be the most valid test to be used in such a setting. Of all the individuals, 13% had less than a 200 μg elastase-1 per gram stool. The prevalence increased with age and was higher in males than in females. The highest prevalence in a subgroup was found in insulin-dependent diabetics (18%). Alcohol consumption was not associated with exocrine dysfunction. In current smokers, the relative risk was greater than 2 when compared to the group that had never smoked. ACE-inhibitor intake, or the disease behind the ACE-use, decreased the relative risk significantly (ACE is also known to increase insulin sensitivity). Taking the possible limitations of this study into consideration, it was concluded that the prevalence of exocrine pancreatic insufficiency (and chronic pancreatitis) must be much higher then previously estimated.
Pathophysiological Concepts
Enrique Dominguez-Munoz (Santiago de Compostela, Spain) discussed the limiting factors regarding “Pathophysiological concepts” of chronic pancreatitis:
• limited knowledge of etiology;
• clinical research is usually limited to advanced.
Genetic, environmental and immunological factors are most probably interrelated and important both as the cause and in the development of chronic pancreatitis. On that basis, Whitcomb et al. have proposed their classification [21]. However, the TIGAR-O classification is 'not' an etiological classification of chronic pancreatitis, but should be looked upon as a 'risk and diseasemodifying factor classification' (Table 4).

Today, there are several hypotheses concerning the cause of chronic pancreatitis:
• toxic-metabolic [22];
• oxidative stress [23];
• stone and duct obstruction [24];
• necrosis-fibrosis;
• primary duct hypothesis;
• sentinel acute pancreatitis event.
Regarding pancreatic stone protein (PSP) much is now known, and its biology has been reevaluated. This protein prevents the precipitation of calcium carbonate but is decreased in the pancreatic juice of chronic pancreatitis [25]. It does, however, not inhibit crystal formation [26] and is not decreased in chronic pancreatitis according to Schmiegel et al. [27]. Moreover, it is not specific for calcifying chronic pancreatitis [28] and there is no PSP-gene mutation in hereditary or idiopathic pancreatitis [29]. Today, the pancreatic stone protein is regarded as a degradation product of the reg protein, a soluble secretory stress protein which is upregulated early in acute pancreatitis. The PSP is converted into insoluble protein aggregates which are resistant to enzyme degradation, but it stimulates pancreatic regeneration (beta-cells) [30, 31].
Today there is also much more known about the role of stimulation of pancreatic stellate cells by cytokines such as platelet-derived growth factor (PDGF), IL-1, IL-6, TNF-alpha and TGF-beta1 [32, 33]. TGF-beta1 plays a central role in pancreatic fibrogenesis and is overexpressed in fibrotic areas of chronic pancreatitis [34]. Pancreatic fibrosis has been demonstrated in TGF-beta1 transgenic mice [35] and cytokines play a key role in matrix remodeling and healing after acute pancreatitis [36].
All this and more can be read in a new book [37].
David Fine (Southampton, United Kingdom) discussed the “Role of pancreatic stellate cells” (PSCs). Increasing evidence suggests that PSCs are the major mediators of pancreatic fibrogenesis and the predominant cell type for matrix synthesis in the pancreas. The pancreatic stellate cells have been shown to be morphologically similar to hepatic stellate cells, the principal effector cells in liver fibrosis. The pancreatic stellate cells are situated at the base of the pancreatic acini, with their extended cytoplasmatic processes encircling the basal aspects of acinar cells. In the healthy pancreas, the stellate cells exist in a quiescent state and can be identified by the presence of vitamin A-containing lipid droplets in the cytoplasm and by immunostaining for cytoskeletal proteins such as desmin and glial fibrillary acidic protein (GFAP). Electron microscopy reveals a prominent rough endoplasmatic reticulum, collagen fibrils, and vacuoles (lipid droplets) surrounding a central nucleus.
Stellate cells, sometimes referred to as differentiated pancreatic myofibroblasts, contain distinctive intracellular lipid droplets and have also been isolated from the rat pancreas. During culture and in response to injury, these cells transform into an activated, myofibroblast-like state. In this activated state, the stellate cells lose lipid droplets, develop long cytoplasmic processes, express the cytoskeletal filament alpha-smooth muscle actin (a marker for cells of smooth muscle origin), and synthesize collagens type I and III, fibronectin, and laminin. In vitro, the stimulation of pancreatic stellate cells can be shown to increase the deposition of extracellular matrix. As such, the stellate cells probably represent the wound-healing myofibroblasts of the pancreas.
Fine emphasized the fact that there is a continuum from the myocytes in the gut to the fibrocyte of the skin, and the stellate cells of the pancreas are somewhere in between. His hypothesis is that in chronic pancreatitis, tissue repair becomes deregulated, resulting in the continued activation of on-going collagen deposition by pancreatic stellate cells. Tissuerepair, however, must be looked upon as a whole-organ process which needs to be directed at the injured tissue and kept out of the healthy tissue - and this might be where the process fails in pancreatic desmoplasia. Repair should, therefore, be self-terminating with the restoration of normal tissue. Chronic pancreatitis probably results from a deregulation of this repair process.
Experimentally, it has been shown that in vitro pancreatic stellate cells enter a stable chronic scarring phenotype which may model the situation in advanced chronic pancreatitis. This is at least partly mediated by the autocrine secretion of TGF-beta.
Hereditary Pancreatitis
The first recognized hereditary pancreatic cancer-prone syndrome was hereditary pancreatitis. It is a disease of the cationic trypsinogen gene, PRSS1. Trypsin and trypsinogen together account for about 30% of the pancreatic secretory proteins in humans. Trypsinogen is activated into trypsin upon cleavage of the activation peptide by enterokinase when it enters the duodenum. The newly formed trypsin plays a central role in food digestion by acting as the trigger enzyme which leads to the activation of all other pancreatic digestive zymogens, as well as trypsinogen itself. There exist several functional trypsin genes which are present on different chromosomes. A deficiency of one isoform can be functionally compensated by others, and a simultaneous loss of all the functional genes is either unlikely or lethal. In contrast, an excess of trypsin is possible and would probably be harmful.
Hereditary pancreatitis is a rare autosomal dominant subgroup of chronic pancreatitis with about 80% penetrance; it was first described as a genetic disease in Minnesota in 1952 by Comfort and Steinberg [38]. Traditionally, the diagnosis was based on the recognition of pancreatitis, usually starting in childhood, in two or more members of a family, in the absence of other known causes of pancreatitis. However, the phenotype can vary even within the same family. The disease is characterized by the early onset of recurrent episodes of acute bouts of acute pancreatitis eventually leading to severe chronic pancreatitis which equally affects males and females. Diagnostic criteria for hereditary pancreatitis have been described, but the clinical and pathologic appearance of both acute and chronic pancreatitis is indistinguishable from the sporadic forms. On the other hand, this suggests that the mutated genes produced in hereditary pancreatitis represent a central and critical component of the mechanism which normally protects others from bouts of acute and chronic pancreatitis.
Roland H Pfützer (Mannheim, Germany) reported that genetic variability at different pro- and anti-inflammatory cytokine loci appears to determine the course of hereditary pancreatitis. Among the anti-inflammatory cytokines, chemokines and neurokines in acute pancreatitis, C5a, cGRP, interleukin 10, IL-1ra and MCP1 should be mentioned and, among the pro-inflammatory, GRO, ICAM-1, IL-1beta, IL-6, IL-8, sIL-2r, PAF, iNOS, substance P and TNF-alpha. However, the precise role for each has not yet been settled, and, for example, regarding IL-1ra, an increased frequency of the IL1RN1 allele was found in patients with severe disease in one study [39] whereas no differences were found between severe and mild cases and controls in another study [40]. For TNF-alpha, no overall association with acute pancreatitis was found [40, 41], but there was an association with septic shock [42, 43] and an association with severe acute pancreatitis [44]. Taken together, it seems, at present, that genetic variability at different pro- and anti-inflammatory cytokine loci appears to determine the disease 'severity' of acute pancreatitis rather than disease 'susceptibility', whereas cytokine polymorphisms may play a minor role for the development of chronic pancreatitis.
There were also three talks on the specific aspects of pancreatitis: acute biliary, obstructive and chronic small-duct pancreatitis.
The Impact of the Composition of Gallbladder Bile and Stones in Biliary Pancreatitis
Dieter Jüngst (Munich, Germany) discussed the “Impact of the composition of gallbladder bile and stones in biliary pancreatitis”. Pigment stones contain less than 10% cholesterol. Non-cholesterol factors in bile (protein, mucines) contribute to the gallstones. With pigment stones, the gallbladder contractility is normal, which means that these stones are already expelled when they are small. There is never a recurrence of acute pancreatitis after a cholecystectomy for pigment stones.
Jüngst had investigated 190 consecutive patients with symptomatic gallstones treated mainly by laparoscopic cholecystectomy: 137 women (age 16-91 years) and 53 men (age 19-88 years); 168 without pancreatitis and 22 with previous biliary pancreatitis. He found that the prevalence of biliary pancreatitis is not affected by the composition of the gallbladder bile. However, the risk of biliary pancreatitis is approximately three-fold higher in patients with pigment stones as compared to patients with mixed or cholesterol stones (which might be caused by the size of the stones rather than the composition).
The Concept and Implications of Chronic Pancreatitis as a Small Duct Disease
Phillip P Toskes (Gainesville, FL, USA) had the task of discussing “Concept and implications of chronic pancreatitis as a small duct disease”. Toskes does not believe that chronic pancreatitis is 'one' disease, but is instead a combination of 'several' diseases with a common histopathology. Regarding large-duct disease and small-duct disease, there are several important differences. In large-duct disease, there is a male predominance, and the secretin test is usually abnormal and serum trypsinogen is often abnormal. There is often a diffuse pancreatic calcification on plain abdominal films and changes verified by ERCP are frequent. The progression to steatorrhea is common, and medical therapy for pain gives a poor to fair response. However, surgical therapy is sometimes helpful. In small duct disease, there is a female preponderance; a secretin test is abnormal, serum trypsinogen is usually normal and calcifications on plain films are infrequent. ERCP-findings can be described as minimal abnormal to normal. There is seldom a progression to steatorrhea, and pancreatic enzymes may give a good to excellent response. Surgery is usually not indicated.
Very elevated CCK levels are found in small duct chronic pancreatitis even at fasting, and increase even more after food. Moreover, 25 of 56 patients with painful small duct chronic pancreatitis (44%) had concomitant gastroparesis [45].
When performing functional tests, it must be remembered that the secretin test is only a measure of bicarbonate secretion by the pancreas. However, there is at least one Japanese study showing a good correlation between histology and secretin. About 40% of patients with a normal pancreatic function test have an abnormal EUS. However, there is little long-term follow-up of isolated EUS changes. Maybe this is a sign of small-duct disease.
As a concomitant part of small duct disease, gastroparesis is a common sign, but it is also a sign which is difficult to treat as most analgesics further induce slowed down motility. However, tramadol does not slow down gastric emptying.
There are also patients with Crohn’s disease with increased CCK levels.
Pancreatic enzymes have been tried as a treatment for pain in chronic pancreatitis (Table 5).
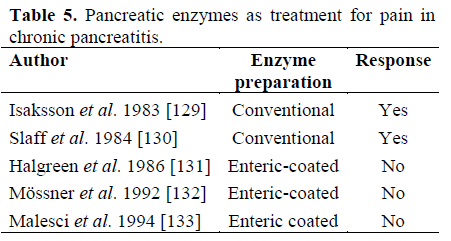
On this basis, Toskes proposed that the type of preparation was important. He stated that the conventional preparation releases its proteases into the duodenum, which is not the case with the enteric-coated preparation. This means that only the conventional capsules may decrease the CCK-levels and decrease the pancreatic secretion - which is the basis for this treatment.
Placebo injections administered three times a day gave increased well-being and less pain in 30% of patients with chronic pancreatitis. Octreotide given in a dose of 200 μg three times daily worked better, whereas 100 μg was without significance. Now, long-acting octreotide has been tried, but so far only 25 patients have been included in that study.
Hans Ulrich Klör (Giessen, Germany) discussed chronic obstructive pancreatitis as a frequent disease related to papillary stenosis and cholelithiasis. He emphasized that cholesterol crystals specifically activate the complement system, attract macrophages and induce the growth of the smooth muscle cells. When implanted anywhere in the body, cholesterol crystals induce connective tissue ('scar tissue') formation. This means that papillary stenosis can be regarded as a disease process analogous to plaque formation in the coronary arteries (The Plaque of the Gastroenterologist).
The exocrine and the endocrine pancreas are sensitive to obstruction at the papilla of Vater (papillary stenosis) leading to chronic pancreatitis. This leads to treatment and/or prevention recommendations:
• primary prevention by the reduction of biliary cholesterol load and lithogenic index;
• aggressive lipid-lowering therapy;
• bile acid therapy;
• secondary prevention by decrease of obstructive damage to the pancreatic ductal system;
• elimination of papillary stenosis as an obstructive mechanism
Tetsuro Kobayashi (Tamaho, Japan), discussed autoimmune pancreatitis from a Japanese perspective. From a clinical point of view, this type of pancreatitis is characterized by:
• T-cell infiltration around the pancreatic ducts with extensive fibrosis of the exocrine pancreas;
• a high prevalence of diabetes;
• histopathological evidence of primary beta-cell damage;
• reversal of exocrine and endocrine dysfunction with corticosteroid treatment;
• beta-cell neogenesis from pancreatic ductal cells.
The diagnostic criteria of autoimmune pancreatitis according to the Japan Pancreas Society (JPS) [46] consist of:
1. Imaging studies: diffuse narrowing of the main pancreatic duct with an irregular wall (more than 1/3 the length of the entire pancreas) and enlargement of the pancreas;
2. Laboratory data: abnormally elevated levels of serum gamma-globulin and/or IgG, or the presence of autoantibodies;
3. Histopathological examination: fibrotic changes with lymphocyte and plasma cell infiltration.
To reach a diagnosis, criterion 1 must be present, together with criterion 2 and/or 3.
T-cell infiltration around the pancreatic ducts with extensive fibrosis of the exocrine pancreas is usual found in addition to autoantibodies against pancreatic exocrine and, occasionally, endocrine cells. In the Japanese study, the following was found:
• anti-nuclear antibody: 76%;
• anti-lactoferrin antibody: 76%;
• anti-carbonic anhydrase II antibody: 59%;
• rheumatoid factor: 29%;
• anti-smooth muscle antibody: 18%.
There is a selective impairment of the betacell area in specimens of autoimmune pancreatitis as compared to type 2 diabetics and controls, which favors immunological processes primarily involving beta-cells. In conclusion, it was stated that [47]:
• immunological processes are primarily involved in beta-cell failure as well as ductal cell destruction in autoimmune pancreatitis;
• diabetes associated with autoimmune pancreatitis can be cured by corticosteroid treatment;
• beta-cell neogenesis from pancreatic ductal cells in autoimmune pancreatitis provide new insights into the tissue stem cells and transdifferentiation research.
Luca Frulloni (Verona, Italy) discussed the European perspective of autoimmune pancreatitis. His clinical group had a 10-year experience (1995-2004) and studied 63 patients (9 with a retrospective diagnosis). With their present knowledge and reputation, they expect 10 new cases per year, which will be about 5% of the pancreatitis patients (Table 6).

Among their 63 patients, 59% had massforming pancreatitis, 19% had 'ordinary' chronic pancreatitis, 19% acute pancreatitis and 3% asymptomatic pancreatitis found at follow-up for ulcerative colitis. In Verona, about 60% of the patients with autoimmune pancreatitis also had another manifestation of autoimmune disease (40% of all had ulcerative colitis and a few had Crohn’s disease). Whether or not this is compatible with two types of autoimmune pancreatitis is still not known. He hypothesized that probably less than 1% of acute pancreatitis, less than 5% of chronic pancreatitis cases (epidemiological data from the PanCroInf- AISP Italian Study in 23 centres and 727 patients [Cavallini G, personal communication]), and 5-10% of pancreatic masses [48, 49, 50] are due to autoimmune pancreatitis. He indicated suspicion of autoimmune pancreatitis if a pancreatic mass was without vascular involvement despite being quite large (greater than 3-4 cm) and if the CA 19-9 was low.
The results of treatment in Verona were also given (Table 7). Three of their patients died (6%) during follow-up; two died from pancreatic cancer 18 and 13 years after diagnosis and one died from adenocarcinoma of uncertain origin after 13 years.

Matthias Löhr (Mannheim, Germany) showed that the histological hallmarks of autoimmune pancreatitis are lymphocytic infiltrates:
square-shaped surrounding ducts, and duct destruction. However, there are no calcifications. From a historical point of view, the circulating antibodies are important [51, 52, 53]. Serologically, today there are autoantibodies against:
• carbonic anhydrase type II (CA II);
• antinuclear and smooth muscle (ANA, ASMA);
• lactoferrin;
• rheumatoid factors.
Serologically, it is also typical that the IgG4 level is elevated [54] and that there is a problem regarding growth factors [55]; for example, there is a defect in TGF-betasignaling. Cellularly, it has been found that CD8+ and CD4+ levels are elevated (i.e. a Th1-immune response) [56]. The first symptom may be obstructive jaundice with a ‘tumor’ in the pancreatic head. Specific fibrotic inflammation and infiltration of IgG4+ cells are seen in many organs, such as:
• (pancreatic) vessels;
• bile ducts;
• salivary gland;
• (cervical) lymph nodes.
Of certain interest are the rather specific - though uncommon - fissural (linear) gastric ulcers perpendicular to the incisura of the lesser curve together with erosive gastritis. Abundant IgG4 with positive immunostaining in plasma cells is present in the gastric mucosa. These signs improve with therapy. Specific inflammation in the pancreas may extend to the papilla and represent the 'only' manifestation in the pancreas or the body.
Relevant pancreatic enzymes are absent in autoimmune pancreatitis:
• proteases (trypsinogens);
• PSP;
• elastase.
From a pathologist’s point of view, Alan K Foulis (Glasgow, United Kingdom) stated that, in autoimmune pancreatitis, the pancreas is diffusely enlarged with an irregularly narrowed duct. Pancreatic cysts, pseudocysts and calcifications are rare, but there is diffuse fibrosis and inflammation: i.e., venulitis.
Concepts of Autoimmunity in Type 1 Diabetes Mellitus
Hubert Kolb (Düsseldorf, Germany) discussed autoimmunity in diabetes mellitus, even though it is still not known today if diabetes type 1 is due to:
• autoimmune beta-cell destruction;
• chronic infection of beta-cells;
• other causes (microbial or sterile) leading to the preferential death of the beta-cells.
Since the presentation of Foulis in 1986 [57], it has been known that the inflammation in diabetes type 1 is driven by insulin-containing beta-cells; without insulin, there is little inflammatory reaction detectable in the islets. However, a case of development of type 1 diabetes despite severe hereditary beta-cell deficiency has also been reported [58].
Another interesting question is why type 1 diabetes affects only beta-cells and not alpha-, delta-, and pancreatic polypeptide (PP)-cells or exocrine cells. The focus of beta-cell autoimmunity on a few phylogenetically conserved antigens fits, however, with the role of phylogenetically conserved receptors of innate immunity. A hypothesis is that heat shock proteins direct the innate immune system towards the beta-cells. This may depend on the fact that heat shock protein contains homologous regions for the key antigens. It has been proposed that heat shock proteins select autoantigenic peptides for MHC presentation. Kolb showed experimental indications that the innate immune system may be crucial for guiding Tcell immune reactivity towards a limited number of autoantigens (and activation of autoimmune T-cells). It can also be mentioned that there are other autoimmune diseases which also depend on a single main autoantigen (Table 8).

Clemens Jaeger (Giessen, Germany), from the planning team, discussed “Autoimmune recurrence in type 1 diabetes - lessons from prevention and islet transplantation studies”. For primary prevention, he stated that the target must be the reduction of the 'incidence' by controlling the etiology and risk factors. To reach this target, the pathogenesis must be established and the risk factors identified. For secondary prevention, the target is the reduction of the 'prevalence' by early, preclinical diagnosis and early therapeutic intervention. A prerequisite for this is a long preclinical phase of the disease, precise prediction models for early diagnosis, and therapeutic interventions which are presumed to be effective.
In a US study starting in 1994 and including 90,000 first degree relatives to diabetics, islet cell antibodies (ICA) were positive in 3,125. The high risk individuals (n=339) were given insulin subcutaneously and the moderate risk individuals (more than 2,500) were given insulin orally. However, the results have been negative so far with regard to a preventive effect of insulin [59].
In Europe, the European Nicotinamide Diabetes Intervention Trial, randomized and placebo controlled, 40,000 individuals were screened between 1993 and 1998, but the results of the intervention are negative [60].
Important lessons from transplantation in patients with longstanding type 1 diabetes are that there are typical surrogate markers characteristic for type 1 diabetes-associated autoimmunity. Moreover, classical immunosuppression appears to have only a moderate effect on these surrogate markers and presumably on the underlying autoimmune process. Therefore, to what extend autoimmunity may contribute to islet graft failure still has to be defined. On the other hand, certain patterns of autoantibodies and cytokines may predict islet graft outcome and may help to develop new strategies to overcome the problem of disease recurrence after transplantation.
Matthias Löhr (Mannheim, Germany) put forward a hypothetical question: “Does exocrine inflammation induce exocrine and endocrine autoimmunity?” He started by stating that in the normal pancreas, there is 'no' epithelial cell/mucosa-associated immune system (MALT). The normal pancreas is also free of resident immunocompetent cells, which means that the detectable immune competent cells are derived from circulation and associated with the vessels.
It is interesting to observe that all cell types in the pancreas can be seen as the main focus of specific diseases (Table 9).

It should also be noted that pancreatitis is also seen in inflammatory bowel disease: 1% (6/553) of patients with pancreatitis [61] and in 30% (3/10) of patients with exocrine pancreatic insufficiency reduced fecal elastase [62]. However, there are no prospective studies.
According to Alan K. Foulis (Glasgow, United Kingdom), autoantibodies to lactoferrin and carbonic anhydrase II are found in approximately 70% of type 1 diabetic patients. These antibodies are not found in type 2 diabetic patients or controls [63]. However, lymphocytic infiltration of the exocrine pancreas is found in 47% of the pancreases of type 1 diabetic patients [64].
Pathologist Alan K. Foulis (Glasgow, United Kingdom) reviewed the histology of the pancreas in chronic pancreatitis and diabetes mellitus. From this review we can see that all Langerhans islets are not the same. For example, in the pancreatic tail, the PP-rich Langerhans islets are bigger and more diffuse (15-500 μm in diameter) than in the head of the pancreas, where the islets are PP-poor. In diabetes, there is a two thirds reduction in weight of the PP-poor lobe whereas the PPrich lobe is unaffected. No difference in overall weight between diabetic and agematched control pancreases are found. The mass of epithelial tissue is not decreased in the PP-rich lobe whereas there is a 30% reduction in the mass of epithelial tissue in the PP-poor lobe. Moreover, there is fibrosis in the body and the tail of pancreas (PP-poor lobe) but not in the head (PP-rich lobe). There is no correlation between the age or the duration of the diabetes and the degree of adiposity of the gland or the degree of fibrosis. The morphology is quite different from chronic pancreatitis.
Insulin is a trophic hormone for the exocrine pancreas, increasing protein synthesis and cell division in acinar tissue. Glucagon and somatostatin are inhibitory to the exocrine pancreas. Glucagon infusion causes atrophy of the exocrine pancreas. Pancreatic polypeptide inhibits exocrine secretion but increases DNA synthesis in the acinar tissue. There are also insulin receptors on acinar cells. The level of insulin in the capillaries around the islets may be up to 100-fold of that in the general circulation. The density of insulin receptors on acinar cells is estimated to be twice that of hepatocytes and there is no obvious down-regulation of receptor density.
Type 1 diabetes is an organ-specific autoimmune disease, where 85% of the patients have islet cell antibodies at presentation. Even though the disease may be seen clinically as an emergency event, betacell destruction takes place over a prolonged period of time. Diabetes develops when 80% of the beta-cells have been destroyed. Therefore, type 1 diabetes patients have three concomitant populations of islets (Table 10).

It is interesting to find that only the beta-cells are destroyed. Most probably, this may be explained by autoimmune targets, but this is still mostly speculation.
There is also a non-autoimmune type 1 diabetes which is a rare disease mostly described from and found in Japan:
• acute presentation with ketoacidosis;
• absent autoantibodies;
• elevated pancreatic serum enzymes;
• diffuse lymphocytic infiltration of the exocrine pancreas;
• insulitis which is not obvious;
• no evidence of chronic pancreatitis.
Type 2 diabetes is part of metabolic syndrome X, including increased insulin resistance. This type of diabetes is not obviously autoimmune.
These patients have amyloid in the islets, a slight reduction in beta-cells (approximately 25%) and a slight increase in alpha-cells (approximately 50%).
There is an exocrine insufficiency in diabetes type 1 and 2 with a reduced fecal elastase in 56% of type 1 patients and in 35% of type 2 patients, but in only 18% of controls. The exocrine pancreatic function in type 1 diabetes is decreased. There is a reduction in enzyme output in 80% of patients. The reduction is related to the duration of the diabetes and the loss of C-peptide secretion. The degree of exocrine abnormality is similar to that of patients with chronic pancreatitis who do not have diabetes. In prolonged duration type 1 diabetes, 20 autopsied pancreases were investigated (duration of diabetes: 3-19 years):
• none had insulin containing islets;
• no evidence of chronic inflammation in any of the pancreases;
• fibrosis (mild periductal) in only one patient (diabetes duration 19 years);
• no evidence of chronic pancreatitis.
There is a reduction in enzyme output in the duodenum in 80% of patients with type 1 diabetes. The reduction relates to the duration of the diabetes and the C-peptide output. This means that pancreatic exocrine malfunction in type 1 diabetes is primarily due to the hormonal imbalance caused by reduced levels of insulin in the capillaries supplying the exocrine pancreas. This may also apply to type 2 diabetes - the exocrine malfunction is less but so is the insulin deficiency.
An interesting model to study these problems is made by injecting alloxane into the pancreatic head for 4 minutes followed by 'antidot' dextrose for 2 minutes, during the clamping of the blood vessels to the tail. This gives an eradication of the beta-cells in the head of the pancreas but unimpaired betacells in the tail. This gives one 'diabetic pancreatic head' and one normal exocrine and endocrine pancreatic head.
Foulis finished by promoting a thrilling hypothesis: insulin containing islets may have evolved to support the massive protein synthesis.
Interaction of the Exocrine and the Endocrine Pancreas, Digestion and Glucose Metabolism
Jutta Keller (Hamburg, Germany) discussed the interaction of the exocrine and the endocrine pancreas regarding digestion and glucose metabolism. The exocrine secretion of the pancreas adapts to the diet, but is also dependent on several other factors. Only about 10-20% of the nutrients entering the duodenum are found in the distal ileum, i.e. they have been absorbed. However, there are considerable differences between the comparable nutrients - for example - rice (little left unabsorbed) and beans (much left unabsorbed).
The normal Western diet contains about 400 g of carbohydrates: 60% starch, 30% sucrose and 10% lactose. The starch is first digested by amylase, mainly from the pancreas, to oligosaccharides (maltose, maltotriose, dextrins) and then by the brush-border enzymes to monosaccharides such as glucose, fructose, and galactose. However, digestion gives very different elevations of blood glucose and insulin levels. For example, in one experiment, potato dumplings gave only half the increase as compared to pizza and white bread. The speed of intake is also important; sipping glucose gave a much lower increase in insulin as compared to a bolus intake.
There is a stimulation of acinar cell growth via the IGF-1 receptor [65] and a potentiation of the enzyme output elicited by hormonal stimulation [66, 67, 68, 69]. Insulin potentiates secretory responses which are also evoked by neural stimulation [70]. However, there is also an 'inhibition' of lipase (80%) and amylase (25%) synthesis on the pretranslational level in rats in vivo [71, 72]. This means that euglycemic hyperinsulinemia inhibits basal but not stimulated enzyme output in healthy humans [73]. Moreover, experimental hyperglycemia inhibits enzyme output in healthy humans (independent of insulin) [73]. On the other hand, GLP-1 and PYY are mediators of the ileal brake [74].
There is also a nutrient-induced regulation of fed motor and pancreatic responses. Duodenal lipids and proteins stimulate pancreatic secretion and the intestinal fed motor pattern. It also inhibits gastric emptying. Moreover, the protein and lipids induce, regulate and integrate the prandial response. There is a small decrease in enzyme output with age, probably less than 20%, but, as the pancreas has a big functional reserve, this should have no clinical effect on digestion in healthy individuals.
Pancreas Morphology and Pathophysiological Concepts of Exocrine Insufficiency in Diabetes Mellitus
In a normal pancreas, there are about 1 million islets, i.e. 1% of the total pancreatic tissue amount. In each islet there are about 300 cells, 75% of them beta-cells. In each beta-cell, there are about 10,000 granules, each of them containing 200,000 insulin molecules in a single crystal. The principal endocrine function of the pancreas is the regulation of blood glucose levels by cells in the islets of Langerhans. The majority of islet cells are insulin-secreting beta-cells which occupy the center of the islet and also secrete amylin. Located at the periphery of the islet are smaller populations of glucagon-secreting alpha-cells and very small numbers of gamma-cells and delta-cells which secrete pancreatic polypeptide hormone and somatostatin, respectively [75].
Pancreas morphology and pathophysiological concepts of exocrine insufficiency in diabetes mellitus was discussed by Reinhard G Bretzel (Giessen, Germany). Today, there are 6 million diabetics in Germany (300,000 of them with type 1 diabetes); 1.6 million of them are insulin-dependent. This is an enormous health burden both for the individuals and for society - not to mention complications. The incidence of new complications per year in Germany is given in Table 11.

According to Bretzel, the diabetic pancreas is smaller than in healthy controls, mainly due to an involution of the exocrine parenchyma [76, 77, 78, 79]. Moreover, there is acinar fibrosis and pancreatic atrophy [80], and atrophy, fatty infiltration, fibrosis, and loss of acinar cells [81, 82, 83, 84, 85]. About 50% of diabetics have pancreatic fibrosis and the pathological findings of the exocrine tissue are twice as high as in controls [86].
Using direct function tests, the exocrine function is impaired in patients with type 1 diabetes (Table 12). In type 2 diabetes the exocrine function may also be impaired, but the results have been less convincing (Table 12).

According to Hardt et al., in both type-1 and type-2 patients, the exocrine function was also impaired when measured by fecal elastase-1 and chymotrypsin (Table 13) [87].

According to Hardt et al. the prevalence of pancreatic exocrine dysfunction using elastase-1 in 1,015 diabetic patients was mildly impaired in 18% (elastase-1: 100-200 μg/g) and severely impaired in 23% (elastase- 1: less than 100 μg/g) of the patients [88]. Two more studies reported similar results in 112 patients with type 1 diabetes [89] and in 544 patients with type 2 diabetes [90]. However, there is still an ongoing debate as to the reliability and the accuracy of fecal elastase-1 estimations for detecting exocrine pancreatic insufficiency [62, 91, 92, 93, 94, 95].
Bretzel concluded by presenting his pathophysiological concepts and hypotheses of exocrine insufficiency in type 1 and type 2 diabetes:
• insulin has a trophic effect on pancreatic acinar tissue (insulin-acinar portal system) and a lack may cause pancreatic atrophy [96, 97] - insulin also has anti-apoptotic effect on brain cells in Alzheimer’s disease;
• islet hormones have regulatory functions on exocrine tissue which may be impaired [80, 98];
• diabetic autonomic neuropathy may lead to impaired enteropancreatic reflexes and exocrine dysfunction [99];
• diabetic angiopathy may cause local microangiopathy followed by pancreatic fibrosis and atrophy [86, 100];
• elevated hormone and peptide concentrations (glucagon, pancreatic polypeptide P, somatostatin) may suppress exocrine function [97, 101, 102, 103, 104];
• diabetic acidosis may induce mild pancreatitis [105];
• viral infections or autoimmunity may cause damage to both exocrine and endocrine tissues [106, 107, 108, 109];
• cytokines such as TGF-beta1, TGF-alpha and TNF-alpha, gastrin and low reg gene concentrations may interact and further impair exocrine and endocrine functions, respectively [97, 108, 110].
Impact of Free Fatty Acids on Beta Cell Function
Günther Boden (Philadelphia, PA, USA) spoke on the “Impact of free fatty acids on beta cell function”. It has been known for at least 40 years that free fatty acids increase the insulin response, as a direct, rapid effect. The higher the infusion of fatty acids, the more this effect is due to a decreasing insulin clearance (not only increased insulin secretion). Free fatty acids also potentiate glucose-stimulated insulin secretion in young, healthy individuals.
However, long-term effects are more controversial. There is a hypothesis - originally from Sweden [111] - on beta-cell lipotoxicity. It hypothesizes that all patients with chronically elevated plasma free fatty acids (almost all obese individuals) will develop type 2 diabetes. However, only about 20% of obese people will, in clinical practice, ever develop type 2 diabetes mellitus, but it is now well-accepted that increasing free fatty acids increases insulin levels in the blood whereas lowering free fatty acids decreases insulin levels.
Increasing evidence suggests that, in healthy individuals, long-term elevations of plasma free fatty acids within the physiologic range (about 700-1,500 mM) stimulate insulin secretion. Also, current evidence indicates that, in prediabetic patients with impaired glucose tolerance or type 2 diabetes, the longterm elevation of plasma free fatty acids impairs glucose-stimulated insulin secretion.
According to Boden, the currently available data suggest that:
• in healthy individuals, physiologically elevated levels of free fatty acids stimulate insulin secretion (acutely and long-term) precisely to the degree needed to compensate for the free fatty acids needed to induce insulin resistance;
• in individuals, genetically predisposed to developing type 2 diabetes (pre-diabetics), the free fatty acid stimulation of insulin secretion is not sufficient to fully compensate for free fatty acid-induced insulin resistance;
• it cannot be excluded, however, that very long exposure to high free fatty acid levels may result in beta-cell failure in healthy individuals.
From a phylogenetical point of view, some thousands of years ago, humans ate very few carbohydrates, whereas they sometimes had the possibility as hunters to eat a lot of fat - so, perhaps, it cold be that, human metabolic systems are built for fat and not for carbohydrates.
Implications and Possible Clinical Consequences of Exocrine Insufficiency in Diabetes Mellitus
Philip D Hardt (Giessen, Germany) spoke on the “Implications and possible clinical consequences of exocrine insufficiency in diabetes mellitus.” In acute pancreatitis about 50% of the patients have impaired glucose tolerance during the acute phase and 1-5% have persisting diabetes [112, 113]. The prevalence of diabetes mellitus in chronic pancreatitis is 40-70% and in chroniccalcifying pancreatitis up to 90% [114]. Pancreatic diabetes is believed to account for 0.5-1.15% of all patients with diabetes mellitus [115, 116].
The definition of chronic pancreatitis includes persisting or progressive changes of the exocrine function and morphology. A significant number of diabetes patients not only show functional changes, but morphologic changes matching the diagnostic criteria for chronic pancreatitis. A pathologic pancreatic duct morphology at ERCP was found in 40% of patients with insulindependent diabetes (n=43), 58% of ICApositive non-insulin-dependent diabetes (n=12) and in 9% of ICA-negative noninsulin- dependent diabetes (n=22) [117]
In a study by Hardt et al., ERCP was done in 150 diabetic patients (Table 14) [118].

Then the question is: how can type-3 diabetes mellitus be that frequent if the incidence of chronic pancreatitis is believed to be as low as 0.2 to 8 per 1,000 inhabitants in clinical studies [119, 120]? The answer must be that chronic pancreatitis and type-3 diabetes mellitus are underestimated in clinical studies as compared to autopsies. In 3,821 autopsy cases, 5.3% of non-diabetics had chronic pancreatitis, but 11.2% of diabetics had chronic pancreatitis [121]. In a study of 394 autopsy cases, Olsen et al. [122] found that only 2 cases (0.5%) had been diagnosed with clinical chronic pancreatitis, but 13% actually had chronic pancreatitis. Of patients with chronic pancreatitis at autopsy, 19% had clinical diabetes, whereas 7% of cases without chronic pancreatitis at autopsy also had clinical diabetes. This underestimation in the clinical praxis is probably due to the following reasons:
• symptoms of exocrine disease are not specific in the early stages of chronic pancreatitis;
• since diagnostic procedures have historically been rather invasive (ERCP, direct function tests), their use has been restricted to obvious indications;
• endocrine dysfunction should be diagnosed early and might be the first symptom recognized by patients and physicians.
In the Medical Department of Giessen University Hospital, Germany (a Department with a documented interest for both diabetes and chronic pancreatitis) in 2003-2004, the codes for diabetes 'and' chronic pancreatitis were used in 6.9% (n=160) of all diabetes cases.
In a German multicenter study of 101 patients (30 type-1 diabetes and 71 type-2 diabetes), fecal elastase-1 concentrations of less than 100 μg/g were used as a cut-off value [123]. Forty percent of these patients had of at least 10 g/day of fat in their stool and 41% had less than 7 g/day of fat in their stool. This means that steatorrhea is common in patients with diabetes mellitus and exocrine insufficiency. An association between the clinical symptoms of exocrine insufficiency and the degree of steatorrhea was also found. In a yet unpublished study, it was shown that, in patients with diabetes mellitus and exocrine insufficiency, steatorrhea can be safely treated by enzyme replacement therapy. Clinical symptoms improved with enzyme therapy. However, the difference was not statistically significant, probably due to the small number of patients.
Studies on enzyme replacement therapy in patients with diabetes mellitus and exocrine pancreatic insufficiency with regard to glucose metabolism showed:
• no positive effect on HbA1c; less stable control in patients with diabetes in chronic pancreatitis [124];
• positive effect on HbA1c and more stable disease in patients with diabetes mellitus in tropical pancreatitis [125];
• no positive effect on HbA1c but more stable control in patients classified as insulinopenic diabetes in chronic pancreatitis [126].
This means that in insulin-treated patients with diabetes mellitus and exocrine pancreatic insufficiency, neither obvious benefit, nor a negative effect regarding the control of glucose metabolism could be observed with enzyme replacement therapy. However, in NIDDM patients with steatorrhea the GIPresponse after ingestion of nutritients is reduced and this might affect the metabolism of the glucose. Substitution of pancreatic enzymes improves digestion and GIP secretion. Insulin response and glucose tolerance are also improved [127]. Furthermore, GLP-1 leads to an upregulation of beta cell mass and proliferation, and reduces beta cell apoptosis [128]. These effects might also be impaired in patients with steatorrhea.
End of the Workshop
After one and a half days of intense discussions Clement W. Imrie (Glasgow, United Kingdom) summarized what had been discussed and the new questions which had been raised:
• emphasis of underdiagnosis of chronic pancreatitis;
• diverse reviews;
• pancreatic polypeptide and pancreatic stellate cells - what is their behavior?
• free fatty acids and proinsulin (C-peptide) - how are they involved?
This particular meeting will change our way of thinking - and maybe working - in the future. Clement W Imrie thanked the Giessen planning team for an excellent workshop. The authors would like to express their gratitude to all the participants of the workshop (Table 15, Figure 2).
