- (2012) Volume 13, Issue 4
Thiruvengadam Muniraj*, Harry R Aslanian
Section of Digestive Diseases, Yale University School of Medicine. New Haven, CT, USA
Received April 20th, 2012 Accepted April 27th, 2012
Context Propofol can cause acute pancreatitis related to hypertriglyceridemia, however, other mechanisms may also exist. Case report A 71-year-old male on propofol infusion in the intensive care setting, developed acute pancreatitis as confirmedbiochemically and by imaging. He did not have any elevation of triglycerides and on propofol re-challenge, had recurrence of pancreatitis with the absence of triglyceride elevation. Conclusion We report a case of hypertriglyceridemia independent propofol induced pancreatitis possibly related to an idiosyncratic reaction and propose that this drug to be reassigned as a class Ia drug as an etiologic factor for acute pancreatitis.
Amylases; fospropofol disodium; Hypertriglyceridemia; Lipase; Pancreatitis; Propofol
We report a case of propofol induced acute pancreatitis in an intensive care patient without elevation of serum triglyceride levels. The acute pancreatitis resolved with discontinuation of propofol and reoccurred with its reintroduction.
A 71-year-old hypertensive male was found to have an acute right middle cerebral artery stroke. After appropriate intervention with a right carotid artery stent, the patient was started on eptifibatide drip, but developed hemoptysis and inability to protect his airway, requiring an intensive care unit admission, intubation, and mechanical ventilation. Propofol infusion was started for sedation and continued for 6 days to a total dose of 8.8 g.
On the fifth day, the patient developed epigastric tenderness and abdominal distension. The patient was afebrile with the Glasgow coma scale of 13. His vitals were mean arterial pressure 68 mmHg, heart rate 106 per min, respiratory rate 14 per min and oxygen saturation of 93%. Laboratory data revealed hematocrit 44% (reference range: 39-54%), white blood cells 11,400 mm-3 (reference range: 3,800-11,000 mm-3), pH 7.34 (reference range: 7.35-7.45), sodium 139 mEq/L (reference range: 135-145 mEq/L), potassium 3.8 mEq/L (reference range: 3.5-5.0 mEq/L), blood urea nitrogen 32 mg/dL (reference range: 6-23 mg/dL) and creatinine 1.2 mg/dL (reference range: 0.6-1.5 mg/dL). Serum amylase and lipase were elevated at 1,062 IU/L and 1,911 IU/L (reference range: 30-110 IU/L and 5-24 IU/L, respectively) with a serum triglyceride level of 102 mg/dL (reference range: 40-150 mg/dL) and a mild elevation in liver transaminases and alkaline phosphatase. The calculated APACHE II risk score was 9 points. Propofol infusion was discontinued and dexmedetomidine was started.
Amylase and lipase levels came down to 100s within 2 days of stopping propofol. Ultrasound of the abdomen showed no gallstones or biliary dilation. CT scan of the abdomen showed an edematous pancreatitis (Figure 1). He had no history of cholelithiasis, cholecystitis, pancreatitis, hypertriglyceridemia or significant alcohol ingestion.
Due to persistent hypertension and agitation, propofol was carefully reintroduced. His amylase and lipase increased to 572 IU/L and 767 IU/L within 24 hours after restarting propofol. The triglyceride level remained normal at 80 mg/dL. Propofol was discontinued and the enzyme levels normalized to 107 and 46 units the next day (Table 1).

Due to the inability to wean off the ventilator, the patient underwent tracheostomy and percutaneous endoscopic gastrostomy for nutrition support on the 17th day and was transferred to a long term care facility.
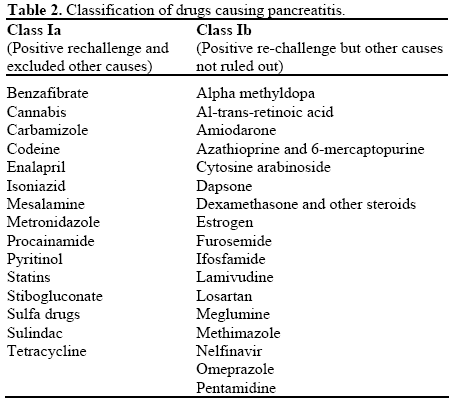
Drug-induced pancreatitis is rare but should be considered in patients who present with idiopathic pancreatitis. Around 100 drugs have been reported to cause acute pancreatitis via various mechanisms. The incidence of drug-induced pancreatitis has been reported to be 1.4% among 1,613 patients treated for acute pancreatitis from all causes [1]. The drugs are classified according to the level of association with acute pancreatitis, evidence of re-challenge, exclusion of other causes, latency of presentation and number of published case reports. The pathogenesis of drug induced acute pancreatitis may be due to intrinsic toxicity or an idiosyncratic reaction, which may be related to accumulation of toxic metabolites or a hypersensitive reaction [2].
In 2007 Badalov et al. presented a classification system with five categories. Class Ia includes drugs with at least one case report, evidence of positive re-challenge and exclusion of other causes of acute pancreatitis. Class Ib is similar to class Ia; however, other causes of acute pancreatitis could not be excluded (Table 2). Class II drugs include drugs in which there is latency in 75% or more of the reported cases. Class III drugs include drugs with two or more case reports published, but neither a re-challenge nor a consistent latency period. Class IV drugs are similar to class III drugs, but with only one case report published [3].
Propofol (Diprivan®, APP Pharma, Schaumburg, IL, USA), abbreviated version of diisopropyl intravenous anesthetic) is a lipid based short acting intravenous hypnotic agent has been available since 1986 as a 1% solution. It is often used for sedation in the intensive care unit as well as induction and maintenance of anesthesia. It is almost insoluble in water and is formulated in a oil-in-water emulsion. Along with 1% propofol, the preparation also contains 10% soybean oil, 1.2% purified egg phospholipid as an emulsifier, 2.25% glycerol and sodium hydroxide. These additional agents are the main constituents in many lipid preparations used for total parenteral nutrition The lipid emulsion of propofol decreases the bacterial clearance and EDTA is added as a chelating agent which also has some bacteriostatic properties. Triglyceride levels higher than 1,000 mg/dL are an identifiable risk factor for the development of acute pancreatitis [4]. In a patient review from 1982 to 1994 from four different hospitals, hypertriglyceridemia was the etiology in 1.3-3.8% of patients discharged with a diagnosis of pancreatitis, with a drug or diet related etiology suspected in one-third [5].
Hypertriglyceridemia can be due to a primary genetic defect in lipid metabolism or secondary to diabetes, obesity, medication use, etc. Propofol being a lipid based emulsion infusion may rarely cause marked hypertriglyceridemia [6, 7, 8, 9, 10]. There are several recent cases of acute pancreatitis attributed to propofol related to hypertriglyceridemia [11, 12, 13, 14]. A prior tolerance of propofol does not exclude the development of pancreatitis in the future [15].
A water-soluble prodrug form, fospropofol, has recently been approved by FDA since 2008 (Lusedra®, Eisai, Woodcliff Lake, NJ, USA). Fospropofol is rapidly broken down by the enzyme alkaline phosphatase to form propofol. Fospropofol may not produce the pain at injection site that often occurs with propofol and it does not have side effects related to a lipid base [16].
This case represents a rare development of propofol induced acute pancreatitis in the absence of hypertriglyceridemia and is the first case with the repeat development of acute pancreatitis with rechallenge and persistently normal triglyceride levels. Kumar et al. reported a case of propofol induced pancreatitis associated with hypertriglyceridemia (triglyceride serum levels greater than 1,400 mg/dL) after total propofol infusion of 26.5g over 7 days. Rechallenge with a dose of 200 mg propofol led the development of acute pancreatitis with no triglyceride elevation, possibly due to dose related toxicity [17]. The development of acute pancreatitis which recurred immediately with rechallenge in the absence of triglyceride elevation in our case would support an idiosyncratic drug reaction as the mechanism, similar to the findings of Donmez et al. [18].
We believe our case, in combination with prior published reports, merits propofol to be reassigned as a class Ia drug. In addition, our findings suggest that hypertriglyceridemia may not be the only mechanism by which propofol may cause pancreatitis, with our case supporting an idiosyncratic hypersensitivity.
Authors disclose no financial relationships relevant to this publication
Nil