- (2006) Volume 7, Issue 4
Charles L Perkins1, Tim Fox1, Eric Elder1, David A Kooby2, Charles A Staley III2, Jerome Landry1
Departments of 1Radiation and 2Surgical Oncology, Emory University School of Medicine. Atlanta, GA, USA
Received: 29 March 2006 Accepted: 8 May 2006
Context Daily setup errors and changes in body habitus during external beam radiotherapy can result in interfraction variation, contributing to uncertainties in treatment delivery. The conventional method of patient positioning using external skin markings is inadequate in reducing these interfraction variations. Objective To evaluate use of on-board imaging with daily kV-kV image matching to reduce interfraction variation in patients with primary gastrointestinal cancer. Patients To evaluate interfraction variation, 13 patients underwent radiotherapy for primary non-metastatic gastrointestinal cancer as did 1 patient with renal liposarcoma. Interventions After conventional external setup, kV-kV image matching was performed using bony landmarks or radiopaque surgical clips with a Varian on-board imager. The degree of shift between the real-time patient position and the planning position were recorded in three planes, and appropriate corrections were made for treatment. Main outcome measures Degree of shift, acute toxicity and local response were assessed. Results For 276 daily on-board imaging sessions, average shift was 0.30±0.42 cm (vertical), 0.33±0.34 cm (longitudinal), and 0.35±0.39 cm (lateral); average 3-D vector shift was 0.71±0.52 cm. Percentage of shift greater than or equal to 0.5 cm was 25% (vertical), 28% (longitudinal), and 30% (lateral); percentage of total vector shift greater than or equal to 0.5 cm was 64%. The pattern of shifts showed a random distribution over time. At median 6-month follow-up, 3 (21%) patients had radiographic local disease regression, ten (71%) had local disease stabilization, and 1 (7%) had local progression. Conclusion Use of daily on-board imaging kV-kV matching reduced uncertainty in amount of dose delivered, potentially resulting in improvement in local control and reduction in treatment toxicity.
Gastrointestinal Neoplasms; Image Processing, Computer-Assisted; Radiotherapy, Computer-Assisted; Radiotherapy, Conformal
CTV: clinical target volume; DRR: digitally reconstructed radiograph; GTV: gross tumor volume; IMRT: intensity modulated radiotherapy; OBI: on-board imaging; PTV: planning target volume; RTOG: Radiation Therapy Oncology Group
During the course of radiation therapy, both the patient’s position and anatomy can vary considerably from the original treatment planning setup. Numerous factors account for this, including changes in patient positioning between fractions (interfraction variation) and those that occur during the treatment fraction (intrafraction variation) [1]. This can result in insufficient dose to the targeted tumor volume and an overdose to critical surrounding normal tissues. Interfraction organ motion has been analyzed for various anatomic sites such as the prostate [2, 3] and intrafraction motion has been evaluated in liver, pancreas, and kidneys [4]. However, there has not been a systematic daily measurement of the internal motion of organs in the abdomen that can result from day-to-day changes, or even motion during radiation treatment.
Interfraction and intrafraction variances are minimized through reproducible patient positioning and artificial immobilization [5]. Simulation and treatment planning take these alterations into account; however, gastrointestinal organs move within the peritoneal cavity during digestion, elimination, and breathing. These uncertainties of tumor volume are routinely accounted for by the use of a set of extra hierarchal margins around a defined clinical target volume (CTV). In the International Commission on Radiation Units (ICRU) Report 62 [6], this planning target volume (PTV) margin is divided into two components: 1) internal margin to account for variations in size, shape, and position; and 2) setup margin to account for uncertainties in patient positioning and beam alignment.
Newer treatment modalities such as intensity modulated radiotherapy (IMRT) are direct attempts to deliver a more conformal dose distribution, resulting in deliverance of a higher therapeutic dose to the defined gross tumor volume (GTV), CTV, and PTV while minimizing the dose absorbed by surrounding critical, normal structures. However, the treatment planning involved in IMRT calculations is inherently dependent on the uncertainties in interfraction and intrafraction variation, and limit the effectiveness of this treatment modality. In fact, studies have shown that blurring of dose distribution can occur more profoundly with IMRT [7]. Furthermore, although studies have examined the amount of margin needed to adequately cover a volume of interest with IMRT in relatively static sites such as the head and neck region [8], the same undertaking has not been reported for more dynamic sites such as the abdomen. We describe our initial results in a group of patients with primary abdominal cancer using kV-kV (kilovoltage) matching with an on-board imager (OBI) from Varian Medical Systems (Palo Alto, CA, USA). Data are presented as part of the ongoing Image Guided Radiotherapy Project at the Emory University Radiation Oncology Department. The goal of this project is to refine and maximize the accuracy of radiotherapy delivery by reducing inter- and intrafraction variances.
Patients
Thirteen patients with primary gastrointestinal cancer and 1 patient with a primary renal liposarcoma status post nephrectomy (10 males, 4 females, mean±SD age; 55.4±13.2, range 34-82 years) were treated at the Emory Winship Cancer Institute Radiation Oncology Clinic from July 2004 through September 2005; details of the individual patients are listed in Table 1. Each patient was simulated using CT-guidance. Eight patients were inversely planned using IMRT on the Eclipse treatment planning system from Varian Oncology Systems; 2 patients were planned using 4D-CT respiratory gating, and treated on three phases of the respiratory cycle corresponding to end expiration. Plans were approved based on optimal dose to be delivered to defined PTVs as well as appropriate dose limitations to adjacent structures. Each patient was also evaluated by the Emory University Medical Oncology Clinic and was treated concurrently with appropriate chemotherapy as determined by that service.
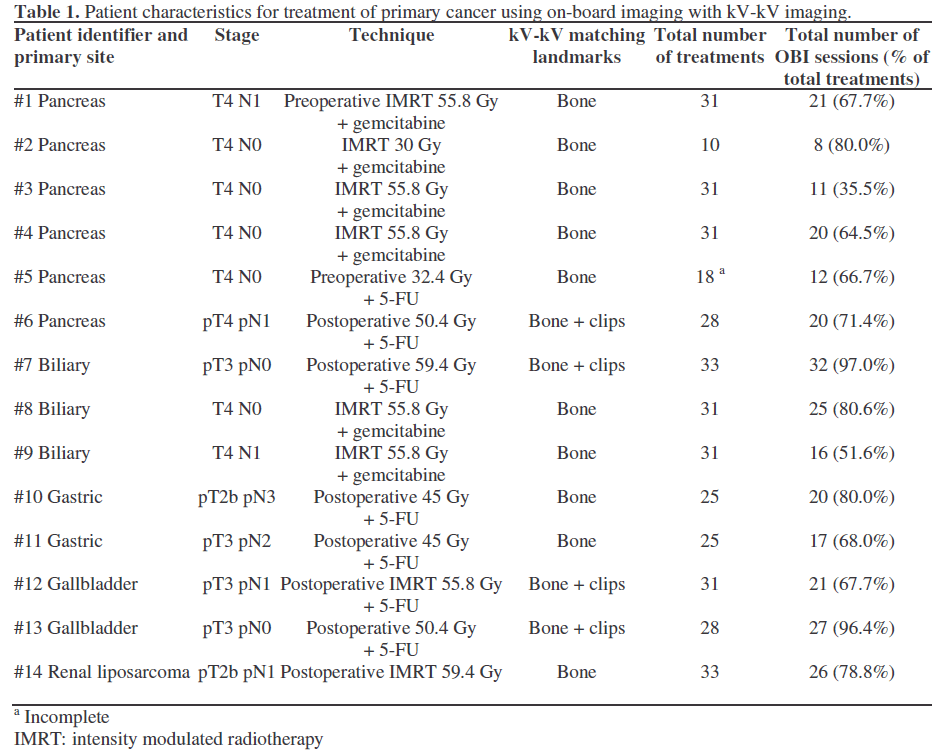
kV-kV Matching
To measure interfraction variation, on selected treatment days each patient was evaluated using a kV on-board imager from Varian Medical Systems (Palo Alto, CA, USA). We did not use kV matching for each treatment session in all patients because of our initial concerns about treatment time. The patient is initially setup conventionally using wall lasers aligned to external skin markings made during initial simulation. Once the patient is positioned, anterior-posterior and lateral kV images are taken using the onboard imager. These kV images are then compared on the OBI software via direct overlay with the digitally reconstructed radiograph obtained from the initial simulation using 2-dimensional matching (Figure 1). Landmarks such as bony anatomy in the abdomen/pelvis or radiopaque surgical clips (Table 1) were used to determine the amount of shift needed to align the patient to the correct position, as determined by the initial simulation. These shifts were measured and recorded in the vertical (anteriorposterior), longitudinal (superior-inferior), and lateral (medial-lateral) dimensions. Manual matching of the images was initially performed, followed by software automatching utilizing a selected volume of interest. Once the degree of shift for each dimension was determined, the patient was aligned into the desired treatment position; 95% of these shifts were performed manually, with the remaining 5% made automatically. Direct physician approval was required for any shift greater than 5 mm. Once the patient was repositioned irradiation commenced as directed by the treatment prescription.
Figure 1. Composite image of a kilovoltage (kV) image (upper right and lower left boxes) obtained on the day of treatment with the digitally reconstructed radiograph (DRR); upper left and lower right) from the CT image at the time of initial simulation. Image matching to bony landmarks or radiopaque surgical clips were made automatically or manually. In this case, the software automatically calculated a shift of 0.2 cm vertically (Vrt), 0.1 cm longitudinally (Lng), -0.6 cm laterally (Lat), and 0.0 cm rotationally (Rtn).
Informed consent for the study was obtained from each patient at the time of simulation. The treatment protocol was approved by the Emory University Human Investigations Committee.
Two hundred and seventy-six sessions using OBI were recorded. The average daily shift and the minimum, maximum, and median values for each dimension were determined by standard calculations. The standard deviation (SD) for each category of data was determined by the squared root of (n Sx2 - (Sx)2) / (n (n-1)). In addition, absolute and relative frequencies were reported and 95% percentiles were computed. Descriptive statistics were computed by means of the Microsoft Excel 2003 for Windows XP. Shifts were compared among different sites by means of the Kruskal-Wallis test. The posthoc pairwise comparisons of each site vs. the others, as well the bone vs. bone+clips shifts, were analyzed by means of the Mann- Whitney test. The SPSS version 13.0 for Windows was used to test data. Two-tailed P values less than 0.05 were considered statistically significant.
Average Daily Vector Shifts
Six patients with pancreatic adenocarcinoma, 3 patients with cholangiocarcinoma, 2 patients with gastric adenocarcinoma, 2 patients with gallbladder cancer, and 1 patient with a post-nephrectomy renal liposarcoma and positive margins were analyzed with daily on-board imaging. Match points were based on bony anatomy or postsurgical clips (Table 1). Average daily shifts recorded for vertical, longitudinal, and lateral directions are summarized in Table 2. Rotational shifts from the superior/inferior axis were also recorded, but not further analyzed due to the consistently low value of these errors. Two hundred and seventy-six OBI sessions were recorded among the 14 patients treated; absolute average daily shifts were 0.30 cm (vertical), 0.33 cm (longitudinal), and 0.35 cm (lateral). The standard deviations for the vertical, longitudinal, and lateral shifts were close to the value of each average daily shift. These results produced an average daily 3- dimensional vector shift of 0.71±0.52 cm. The median shift for each dimension was 0.20 cm (vertical and longitudinal) and 0.28 cm (lateral). For all sessions recorded, the maximum absolute shift was 4.0 cm in the vertical direction, 2.3 cm in the longitudinal direction, and 2.4 cm in the lateral direction. For all gastrointestinal shifts recorded the total vector shifts greater than or equal to 0.5 cm were 176 (63.8%). The 95% percentiles of all shifts (vertical, longitudinal, and lateral) and total vector shift were 1 and 1.65, respectively (all shifts less than or equal to 1.0 cm: 787/828, 95.0%; total vector shift less than or equal to 1.65 cm: 263/276, 95.3%).
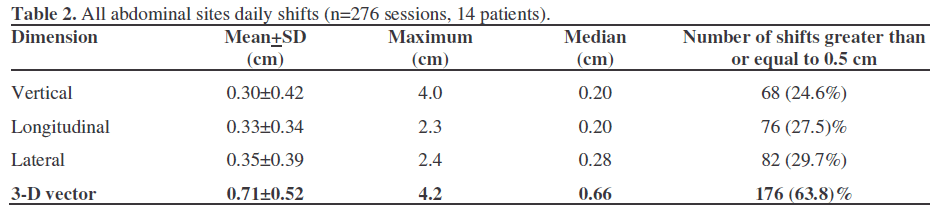
The inclusion of surgical clips as match points showed a statistically significant higher degree of vertical, lateral, and 3-D vector shifts recorded versus bony landmarks, while longitudinal shifts were comparable (Table 3). When grouped by primary site of disease, statistically significant differences were found for the average daily vertical, longitudinal, and lateral shifts (Figure 2), while the 3-D vector did not result in a significant difference among the different sites. In particular, the pancreas showed significantly higher vertical shifts, while longitudinal shifts were significantly lower in the gallbladder and higher in the renal liposarcoma and lateral shifts were significantly lower in the stomach and higher in the gallbladder.
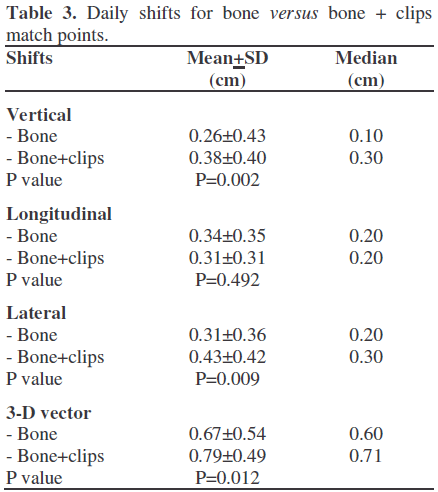
Representative examples from each abdominal site are depicted in Figure 3, portrayed as scatterplots of individual data points. No consistent differences or trends in a single patient with respect to each individual dimension were recorded.
Figure 3. Representative scatterplots of the daily shifts required to correct the patient to the desired treatment position, represented by shifts in the vertical (anterior/posterior), longitudinal (superior/ inferior), and lateral (medial/lateral) directions. Shifts were determined either manually or automatically by the software. Each point represents one observation and each panel shows the treatments of one patient.
At its original inception, 30 minutes of additional treatment time were allocated to each patient’s appointment in order to account for the kV-kV matching. However, after 8 weeks of routine on-board imaging, the amount of time needed to perform the procedure was consistently less than 5 minutes. Thereafter, no additional time was allocated to a patient’s appointment slot for daily on-board imaging.
Acute Toxicity
During the course of treatment, each patient was evaluated on a weekly basis, and the degree of toxicity from treatment was recorded based on the Radiation Therapy Oncology Group (RTOG) grading system. Eight (57.1%) of the 14 patients were treated to their planning target volume using IMRT, and 13 (92.9%) of the patients received chemotherapy concurrently during radiation treatment: 6 with weekly gemcitabine (42.9%) and 7 with 5-FU/leucovorin (50.0%). Toxicity results are recorded in Table 4. One (7.1%) of the 14 patients treated had documented grade 3 nausea and vomiting, whereas the other 13 all had nausea/vomiting and diarrhea ranging from grade 0 to grade 2.
Radiographic imaging, using computed tomography (CT) or magnetic resonance imaging (MRI) was used for evaluation of disease progression. Follow-up data are available for a median of 6 months after treatment with radiotherapy; results are summarized in Table 4. Of the 14 patients treated, 3 (21.4%) had disease regression immediately after treatment, 10 (71.4%) had disease stabilization, and 1 (7.1%) had disease progression. The 3 patients with disease regression each had pancreatic adenocarcinoma, and all showed stabilization of disease up to 15.5 months after treatment. The 10 patients with disease stabilization after treatment included 2 pancreatic adenocarcinoma patients, the 3 cholangiocarcinoma patients, the 2 gastric adenocarcinoma patients, the 2 gallbladder cancer patients, and the post-nephrectomy renal cell carcinoma patient. The pancreatic adenocarcinoma patient with disease progression had developed liver and lung metastases on the first post-treatment imaging, and had not received the complete prescribed radiation dose. This patient halted treatment after receiving 32.4 Gy. Two patients noted transient, late (more than 90 days past radiation treatment) grade 2 nausea and vomiting toxicity; both resolved after completing post radiation chemotherapy.
We report the initial results of utilization of on-board kV-kV imaging to reduce interfraction variations in the daily treatment of abdominal cancers with radiotherapy. The use of a daily kV-kV image matching system allows for a more precise system for patient setup compared to traditional weekly MV (megavoltage) imaging, and also results in a substantial reduction in non-therapeutic dosage to the patient, an unavoidable consequence of high energy MV setup confirmation. Each weekly MV portal image can contribute a dose of 3 to 10 cGy, compared to 0.1 to 0.3 cGy for a kV on board image. For 30 treatments, the reduction in patient dose can therefore be reduced by roughly 85% with the use of kV image guidance. Although the patients in this study did not receive kV on-board imaging at every treatment session, all but 2 of the 14 received matching for greater than 60% of their total treatment sessions. We did not use kV matching for each treatment session in all patients because of our initial concerns about treatment time. The eventual goal is to utilize such a matching system on a daily basis to reduce interfraction variation at each treatment session. This system may allow for adjustments of error in treatment delivery due to setup error, as well as changes in body habitus such as weight loss or ascites, which would not be compensated for in a conventional setup using external skin markings.
The results of this study reveal that with conventional setup, there is an average daily error of about 0.3 cm in each dimension of vertical (or anterior-posterior), longitudinal (superior-inferior), and lateral (mediallateral), resulting in a 3-dimensional average vector shift of 0.7 cm. The use of surgical clips as matching points appeared to result in a statistically higher shift in the vertical, lateral, and 3-D vector dimensions when compared to bony landmarks; longitudinal shifts were similar. This may be the result of intra-abdominal organ and cavity motion. Other studies have shown similar amounts of intrafraction organ motion in the abdomen and pelvis [3, 4, 9]. Implantation of radiopaque markers into the prostate showed an initial displacement of the gland in the anterior-posterior, superior-inferior, and lateral direction of -0.9±3.9 mm, 0.1±3.9 mm, and 0.2±3.4 mm, respectively [3]. This study does not show how such a variation would affect the subsequent daily setup, or whether a compounding of error can occur with each sequential session, because corrections are made at each matching session. We also show that approximately 20% to 30% of the daily errors are at least 0.5 cm for any dimension. If potentially one-third of daily treatments incorporate this error, this can have profound effects on the selection of treatment margins needed to incorporate this error. More telling, however, is that variations as high as 4 cm can occur, albeit at a much lower frequency, which can have significant impact on targeted radiotherapy of the volume of interest and minimizing toxicity due to overdosage of surrounding structures. For instance, recent data from Michigan have resulted in the recommendation of reducing the PTV to the GTV plus a 1-cm margin for the treatment of pancreatic carcinoma with concurrent gemcitabine [10]. If one takes into account interfraction variations (and to a larger degree, intrafraction variations - discussed below), this margin might not be adequate to cover the gross disease volume on a consistent day-to-day setup. Based on the shifts observed using kV-kV matching with OBI, a PTV margin needed to account for daily setup variation in gastrointestinal cancer can be estimated. Without using daily kV setup verification, a margin of 1 cm vertically, longitudinally, and laterally is necessary to account for 95% of the variation observed. The use of OBI can eliminate this degree of error, potentially reducing the PTV margin needed. However, the amount of PTV margin needed to account for intrafraction variation has yet to be adequately determined.
Although each of the patients in this series was similarly set up with external skin markings, there were statistically significant differences seen in the individual dimensions between the various sites examined. However, the overall 3-D vector shift was not different among these sites. As such, there appeared to be no systematic error with daily conventional setup. There was a trend toward a higher mean shift when the first five OBI treatments of the initial four patients were examined (data not shown). These patients were in the first group treated in the clinic using OBI; the average daily shifts of the first five treatments of patients a few months later did not show this trend. This may represent a learning curve in the utilization of new equipment.
One potential drawback of using daily kV-kV matching is the extra time needed to schedule and confirm position and initiate the treatment of the patient. Originally, 30 minutes were allocated for each OBI and treatment session; with frequent usage, the amount of added time to perform kV-kV matching in our clinic does not generally exceed 5 minutes. Currently most sessions incorporating OBI are scheduled in the standard 15-minute treatment allocation slot in our clinic. Simultaneous kV-MV matching was not performed on a daily basis in order to reduce the effective dose given to the patient. Furthermore, the placement of the kV imager is at 90 degrees from the MV gantry, necessitating repositioning for imaging and treatment.
In terms of toxicity, more accurate targeting of the PTV should in theory result in more precise dosage to surrounding critical structures and organs. Although the number of patients in our study is low, and no direct correlation between the use of image guided radiotherapy and acute toxicity can be drawn, this parameter was assessed. In our study, only 1 patient had acute grade 3 nausea and vomiting toxicity during concurrent chemotherapy and radiation; the remainder of the patients had grade 2 or less nausea and vomiting. No patient in our series had more than grade 2 diarrhea.
Reduction in interfraction variation should also result in better targeting of the volume of interest, which may have an impact on local control. In this study, 3 (21%) of the 14 patients had primary disease site regression 1 month after completing treatment; each patient was diagnosed with pancreatic adenocarcinoma. One patient has been followed for 15.5 months after treatment, and has had disease stabilization at the primary site up to that point. The remainder of the patients (except one patient with pancreatic adenocarcinoma, discussed below), have had disease stabilization on radiographic imaging after completing therapy, with a median follow-up of 6 months. The patient with pancreatic adenocarcinoma disease progression 1 month after completing treatment had progression at the primary site as well as failure distally in the liver and the lungs. Treatment was interrupted for this patient before 35 Gy had been delivered; hence the prescribed dose was not received. Thus, there were no local failures among the 13 patients who completed radiation treatment. Again however, given the small number of patients in this study, no direct correlation between the use of on-board imaging and an improvement in local control can be made. Subsequent evaluation with a larger cohort of patients is needed to delineate any significant trend or difference.
One obvious limitation in the use of kV-kV image matching in the daily setup of gastrointestinal cancer is the dependence on bony landmarks for reference points. Another limitation of this technique is that it does not take into account intrafraction variations in the volume of interest. Although kV matching can reproducibly allow for the accurate setup of radiopaque landmarks between fractions, it does not allow for compensation of internal respiratory-related target motion during the treatment cycle itself. One solution is to incorporate daily setup using a cone beam CT for improved soft tissue resolution; this technology is currently under investigation in our clinic.
Although previous studies have shown an internal organ motion of approximately 1 cm [9], preliminary studies in our clinic showed that a significant gross tumor volume shift can occur with respiratory cycling, with movement of up to 1.5 cm in the superior/inferior direction for primaries in the upper abdomen. While implanted fiducials such as metallic markers or surgical clips may offer a more reliable representation of the area of interest intrafractionally, our results indicate that the use of bony landmarks as match points may be slightly superior interfractionally. Whether this results in a clinical difference remains to be determined. Ideally, one would account for interfraction and intrafraction variations simultaneously with a system incorporating OBI and respiratory gating. We have initiated a study examining the feasibility and reproducibility of this technique and our results are forthcoming as part of our image-guided therapy strategy.