Original Article - (2018) Volume 19, Issue 3
Junko Tahara1, Kyoko Shimizu1, Junichi Akao1, Yukiko Takayama1, Katsutoshi Tokushige1, Masakazu Yamamoto2
1Department of Medicine and 2Surgery, Institute of Gastroenterology, Tokyo Women’s Medical University, Tokyo, Japan
Received March 19th, 2018 - Accepted May 16th, 2018
Objective While the long-term prognosis of autoimmune pancreatitis remains unclear, diagnosis of various malignant neoplasms has been reported in several autoimmune pancreatitis case studies. We retrospectively investigated the relationship between type 1 autoimmune pancreatitis and the occurrence of malignant neoplasms. Methods Seventy-eight type 1 autoimmune pancreatitis patients were the subjects of this study. We investigated their clinical profiles, incidence of malignancies, and possible risk factors for developing malignancies, including duration of autoimmune pancreatitis, diabetes mellitus, and steroid therapy. Results Twelve patients developed malignancies after the diagnosis of autoimmune pancreatitis: colon cancer in five patients, lung cancer, pancreatic cancer and gastric cancer in two patients each, and hepatocellular cancer in one patient. In seven of the 12 patients (58.3%) a malignancy was detected within one year after the diagnosis of autoimmune pancreatitis. There were significant differences in gender, median age, and diffuse type of pancreas swelling between the group of patients with malignancies and the group without malignancies, but steroid therapy and diabetes mellitus were unrelated to the occurrence of malignancies in autoimmune pancreatitis. Conclusion The incidence of colon cancer in the type 1 autoimmune pancreatitis patients was higher than the incidence of other malignancies. Male, advanced age, and diffuse type of pancreatic swelling may be risk factors for malignancy in patients with type 1 autoimmune pancreatitis. It is recommended that male autoimmune pancreatitis patients over 70 years old who have been diagnosed with autoimmune pancreatitis within the previous year be examined for malignancy by a combination of gastrointestinal endoscopy, either chest X-ray or computed tomography scan, and abdominal ultrasound.
Neoplasms; Pancreatitis; Steroids; therapy
AIP autoimmune pancreatitis; CT computed tomography; DM diabetes mellitus; ICDC International Consensus Diagnostic Criteria
The concept of autoimmune pancreatitis (AIP) was first proposed by Yoshida et al. [1] in 1995 as a specific from of chronic pancreatitis that responds to corticosteroid treatment. Clinically, AIP is characterized by diffuse pancreatic enlargement and narrowing of the main pancreatic duct. Hamano et al. [2] discovered elevated serum IgG4 levels in AIP patients, and the serum IgG4 levels were sensitive biomarkers of AIP. AIP is classified into 2 types based on its pathological features [3]. Type 1 AIP is characterized by IgG4-positive plasma cell infiltration around the pancreatic duct, obliterative phlebitis, and storiform fibrosis, and type 2 AIP is characterized by granulocytic epithelial lesions (GELs). IgG4-positive plasma cells are not found in type 2 AIP. Extrapancreatic lesions, including sclerosing cholangitis, retroperitoneal The short-term prognosis of type 1 AIP is improved by steroid therapy, and since steroid withdrawal results in AIP relapse [5], in Japan AIP patients receive long-term steroid therapy. In other countries, however, AIP patients have also been treated with immunosuppressant therapy and anti-CD20 antibody therapy [6]. While the long-term prognosis of AIP remains unclear, the development of various malignant neoplasms, including pancreatic cancer [7, 8], colon cancer, gastric cancer [9, 10, 11, 12], and lymphoma [13, 14, 15], has been reported in AIP patients in several case studies and retrospective studies. Although whether AIP is associated with a higher incidence of malignant neoplasms is unknown [16, 17], Shiokawa et al. [18] reported finding that AIP patients are at high risk of various cancers and suggested that they may develop a paraneoplastic syndrome.
In this study, we retrospectively investigated the relationship between type 1 AIP and the occurrence of malignant neoplasms.
Patients
This study was carried out in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Tokyo Women’s Medical University. The cases of 78 patients who were diagnosed with type 1 AIP based on the International Consensus Diagnostic Criteria (ICDC) and Japan diagnostic criteria at Tokyo Women’s Medical University Hospital during the period between April 2000 and February 2015 were retrospectively reviewed to determine their incidence of malignant neoplasms and to identify risk factors. They were followed up regularly, including by performing a range of examinations and collecting clinical data, including serum IgG4 levels and serum tumor marker levels, and computed tomography (CT) examinations. Malignant neoplasms were diagnosed based on the clinical data and pathological evidence.
Malignant Neoplasms of the AIP Patients
We analyzed the characteristics of the AIP patients with malignant neoplasms, including their age and sex, and presence or absence of steroid therapy, diabetes mellitus (DM), the time malignancies were detected, and relapse of AIP after treating the malignancies. We also investigated whether the time when the malignancies were detected was associated with the type of malignancy, serum IgG4 level, relapse of AIP, steroid therapy, family history of malignancies, and diagnostic imaging findings in the pancreas. Since steroid therapy and DM are known risk factors for malignancy, we investigated whether total steroid dose and the presence of DM were associated with the development of malignancies in the AIP patients.
Statistical Analysis
Continuous data were analyzed for significant differences by the χ2 test and Wilcoxon test, and Fisher’s exact test was used for categorical data. The statistical analysis was performed using the JMP version 12.0 software (SAS Institute Inc., Cary, NC). All statistical tests were two-sided, and a p value <0.05 was considered statistically significant.
Patient Characteristics
Twelve (15.4%) of the 78 type 1 AIP patients developed a malignant neoplasm. Their follow-up periods after the diagnosis of type 1 AIP ranged from 959 to 8223 days (Table 1). Their median age was 70 years. Ten patients had received steroid therapy when the malignancy was detected. The median serum IgG4 level of the 12 patients at the time AIP was diagnosed was 535 mg/dl, and the range was 12-2370 mg/dl. DM was present in 7 patients, and 6 patients had a family history of malignant neoplasms. Eight patients had an extrapancreatic lesion other than bile duct stenosis in the pancreas. The diffuse type of pancreatic swelling was present in 9 cases, and the focal type was present in 3 cases. There were significant differences in gender, median age, and type of pancreatic swelling between the group of AIP patients with malignant neoplasms and the group without malignant neoplasms, but there were no significant differences between the group in whether they had received steroid therapy, had DM, extrapancreatic lesions, a relapse of AIP, or a family history of malignancies, or in their serum IgG4 levels. The logistic multivariate analysis showed a significant difference in type of pancreatic swelling (Table1).
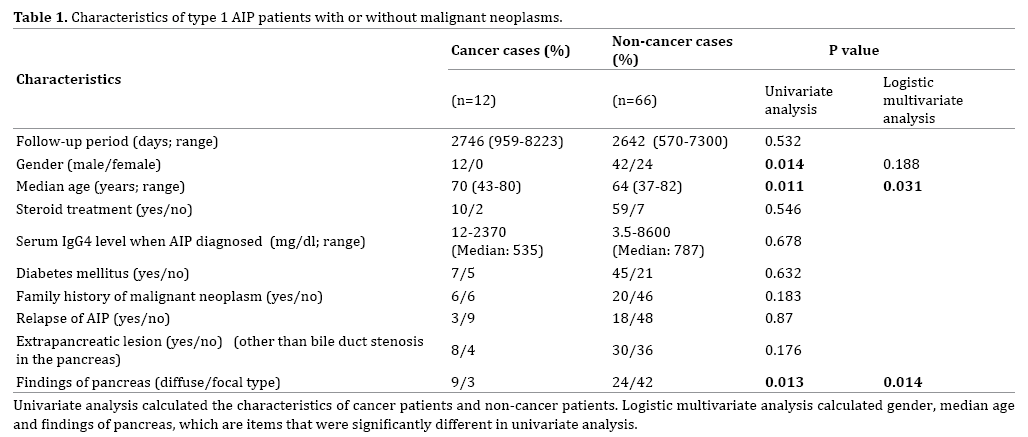
The most common malignancy was colon cancer, which was diagnosed in 5 cases (41.7%), and it was followed by lung cancer, pancreatic cancer, and gastric cancer in 2 cases each (16.7%), and hepatocellular cancer in 1 case (8.3%).
Time the Malignant Neoplasms Were Diagnosed in Relation to the Time Type 1 AIP was Diagnosed
We investigated whether there were differences between the group of patients with malignant neoplasms that had been diagnosed within 1 year after the diagnosis of AIP and the group of patients with malignant neoplasms that had been diagnosed more than 1 year after the diagnosis of AIP (Table 2). Twelve of the 78 AIP patients had developed a malignancy. The malignancy had been detected within a year after the diagnosis of AIP in 7 (9.0%) of the 78 patients, and they accounted for 58.3% of the 12 AIP patients with a malignancy.
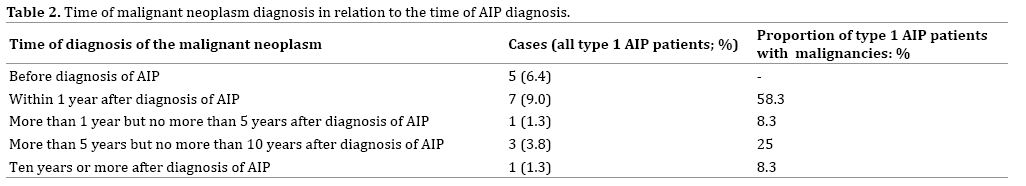
Five of the 78 AIP patients had been diagnosed with a malignancy more than a year after the diagnosis of AIP, and another 5 patients had been diagnosed with a malignancy before the diagnosis of AIP, and thus the 7 AIP patients whose malignant neoplasms were detected within a year after the diagnosis of AIP formed the largest group. We compared the frequency of malignant neoplasms in both the groups. There were 3 cases of colon cancer, and one case each of lung cancer, pancreatic cancer, gastric cancer, and hepatocellular cancer in patients whose malignant neoplasms were detected more than a year after the diagnosis of AIP. There were 2 cases of colon cancer and 1 case each of lung cancer, pancreatic cancer, and gastric cancer in patients whose malignant neoplasms were detected malignant neoplasms more than a year after the diagnosis of AIP. The incidence of colon cancer among the AIP patients was higher than the incidences of the other cancers, and there were no statistically significant differences between the both the groups with respected to types of malignancies.
Treatment of the Malignant Neoplasms and Relapse of AIP after Cancer Treatment
Ten of the 12 patients underwent surgery or endoscopic mucosal resection (EMR) for a malignant neoplasm that was detected after the diagnosis of AIP, and 2 of them had pancreatic cancer. One of the 2 patients with pancreatic cancer received adjuvant chemotherapy after surgery, and the other patient underwent surgery alone. Five patients had colon cancer, and one of them underwent EMR. We discontinued steroid maintenance therapy in four of the 10 patients, but none of them experienced a postoperative relapse. Because of their poor performance status the 2 lung cancer patients could not be treated surgically or by chemotherapy.
Histological Findings and Stage of Malignant Neoplasms
Two of the 5 patients in the present study who developed colon cancer had stage IIIA, and one each had stage IIIB, stage I, and carcinoma in situ. One of 2 patients with pancreatic cancer had stage I, and the other had stage III. One of the 2 patients with gastric cancer had stage I. The one patient with hepatocellular carcinoma had stage III. Both patients with lung cancer had unresectable advanced stage. The pancreatic cancer in both patients was detected in the resectable stage. One pancreatic cancer patient presented with stenosis of the pancreatic duct; the preoperative pathological diagnosis was high-grade dysplasia. Histological findings of the surgical specimen revealed pancreatic cancer cells in the small part of main pancreatic duct, with diffuse inflammatory IgG4-positive plasma cells, lymphocytes infiltration, and obliterative phlebitis in the pancreatic head. The other pancreatic cancer patient presented with atrophic pancreas and infiltrated IgG4-positive plasma cells and lymphocytes around the main pancreatic duct. Cancer cells were detected in the pancreatic head, which metastasized to the lymph nodes.
Total Steroid Dose and Duration of DM
The total steroid dose varied. Three of the 12 patients who developed malignancies after the diagnosis of AIP had not received steroid therapy, and the other 9 patients (75%) had received total steroid doses of 2.9-15.9 g. Nine of the 12 patents had received steroid therapy (Table 3), and maintenance steroid therapy was continued in 8 of the 9 patients. To clarify the effect of steroid therapy on the risk of malignancy, we compared the total steroid doses of the group of AIP patients with a malignancy that had been detected after the diagnosis of AIP and the group of AIP patients in whom a malignancy had not been detected (Figure 1). The differences between the total steroid doses of the two groups were not statistically significant, suggesting that steroid therapy does not promote the development of malignancy and is not a risk factor for malignancy in AIP patients. Finally, we investigated the association between the presence of DM and occurrence of malignancies in AIP patients, but the results showed that the presence of DM was not associated with an increase the incidence of malignancy (Table 1).
Previous studies have reported malignancy prevalence of 10.4%-18% in AIP patients [18, 19, 20, 21], a prevalence range that was consistent with our own findings in the present study, and the most frequent type of malignancy in the AIP patients in the previous studies was colon cancer, which was followed by lung cancer and then gastric cancer. A recent multicenter international analysis revealed that malignant neoplasms had been found in 57 of 978 type 1 AIP patients, and the most frequent malignancies in descending order of incidence were gastric cancer, lung cancer, prostate cancer, colon cancer, and pancreatic cancer [17]. Earlier studies also reported higher incidences of gastric cancer, lung cancer, and colon cancer than pancreatic cancer in Japanese AIP patients [18, 20, 22, 23]. It seems that patients who regularly attend a hospital clinic because of AIP are more likely than healthy subjects to receive gastrointestinal endoscopic examinations and that may have led to early detection of their gastrointestinal cancers.
A malignant neoplasm was detected within a year after the diagnosis of AIP in 9.0% of the 78 type 1 AIP patients in the present study, and they accounted for 58.3% of the 12 AIP patients in whom a malignancy had been detected after the diagnosis of AIP. The incidence of malignant neoplasms tended to be higher within a year after the diagnosis of AIP, and there were no AIP relapses after cancer treatment, suggesting that the development of malignant neoplasms among AIP patients is associated with the occurrence of autoimmune disease.
While it is unclear why malignant neoplasms often develop within a year after the diagnosis of AIP, there are several hypotheses to explain the association between malignancies and AIP. First, chronic inflammatory disease causes malignancies such as colitic cancer in ulcerative colitis, hepatocellular carcinoma in hepatitis C, and pancreatic cancer in chronic pancreatitis [24]. Kamisawa et al. reported detecting more frequent K-ras mutations in the gastric and colonic mucosae of AIP patients as well as in their pancreatic epithelia [25, 26]. Mutationally activated K-ras is the earliest genetic mutation in precancerous lesions and has been found in more than 95% of pancreatic cancer patients and in 27% of chronic pancreatitis patients. Based on these findings, oncogenic K-ras mutations are thought to promote gastric and colon cancer in AIP patients. While K-ras mutation is an early step in the progression toward pancreatic cancer, the incidence of pancreatic cancer was lower than the incidences of other gastrointestinal cancers in the present study as well as in previous studies [18, 19, 20, 23]. Pancreatic intraepithelial neoplasia (PanIN) is recognized as the precursor lesion of invasive pancreatic cancer, and activated K-ras mutations are almost always present in the early stage of PanIN. Gapta et al. have reported detecting PanIN and PanIN 2 in 82% and 25%, respectively, of AIP cases [27]. Their reports support the hypothesis that AIP is associated with an increased risk of malignancy. Pancreatic cancer was diagnosed in 2 of the 78 AIP patients in our study. One of the 2 patients with pancreatic cancer had both a history of three cancers in different organs before the diagnosis of AIP, and a family history of pancreatic cancer, suggesting that genetic factors play a major role in the development of pancreatic cancer. The pancreatic cancer in the other patient was diagnosed 9 years after the diagnosis of AIP, and the patient had received steroid maintenance therapy for 9 years because of repeated AIP relapses. Thus, K-ras mutations and PanIN lesions are likely to be involved in the progression of malignancies in patients with a longer duration of AIP.
The second hypothesis to explain the development of malignancies in AIP is the existence of an immunosuppressed state induced by steroid therapy. Long-term administration of immunosuppressive drugs such as azathioprine has been reported to increase the risk of carcinogenesis [28]. Although 10 of the 12 AIP patients found malignant neoplasms within 1 year after diagnosis of AIP in our study had received steroid therapy, two patients who had not received steroid therapy after being diagnosed with AIP developed malignancies. Although the total steroid doses received by the group of AIP patients with malignancies and the group of AIP patients without malignancies were not significantly different according to the results of a univariate analysis, there were significant differences according to the results of a multivariate analysis. Hirano et al. reported finding that univariate analysis identified age at onset of IgG4-RD >65 years and the presence of DM as significant risk factors for malignancy in patients with IgG4-related disease, but that DM was not found to be a significant risk factor in a multivariate analysis [20]. Consistent with their results, the difference between the incidences of malignancies in the group of AIP patients with DM and the group of AIP patients without DM in our own study was not statistically significant. A study by Shimizu et al. found that 6 out of 9 patients developed a malignancy after the diagnosis of AIP, but steroid therapy was administered after development of the malignancy in all 6 cases. These results suggest that steroid therapy itself does not increase the risk of malignancies in AIP.
A third hypothesis to explain the development of malignancies in AIP is that the immunological response that the malignant neoplasm itself induces promotes the infiltration by IgG4-positive plasma cells. Liu et al. reported observing that IgG4-positive plasma cells had infiltrated pancreatic cancer lesions and the surrounding area and that a high level of intratumoral IgG4-positive plasma cell infiltration was correlated with a poor outcome [29]. Fukui et al. also found that IgG4-positive cells infiltrated pancreatic cancer lesions, peritumoral pancreatitis lesions, and obstructive pancreatitis lesions along with pancreatic cancer and that the number of IgG4-positive cells or the ratio of IgG4-positive cells to IgG-positive cells was higher in the pancreas of AIP patients than of pancreatic cancer patients [30]. They also found a significant correlation between the numbers of regulatory T-cells (T-regs) and IgG4-positive cells in obstructive pancreatitis lesions. A similar immune mechanism appears to be involved in the production of IgG4-positive cells in AIP and in obstructive pancreatitis in pancreatic cancer. We therefore speculated that the development of AIP within a year after the diagnosis of malignancy is related to a cancer-associated immune response and that the development of a malignancy several years after the diagnosis of AIP is associated with chronic inflammation and/or a genetic mutation.
Three of 5 colon cancers occurred within a year after the diagnosis of AIP, and we thought that they are related to the cancer-associated response. On the other hand, the other two of 5 colon cancers occurred after 8 years and 20 years, and chronic inflammation was not found in their surgical specimens. As the incidence of colon cancer was generally high and colonoscopy was frequently performed during screening, we suspect that the number of colon cancer patients in our study was higher than that of patients with our study types of cancer. While it is important to clarify the immune mechanism of type 1 AIP regarding the development of colon cancer, the association between immune mechanism in type 1 AIP and the incidence of colon cancer in this study remain unclear.
In conclusion, although the mechanism underlying the development of malignant neoplasms in AIP patients has yet to be identified, the incidence of malignancies was relatively high within a year after the diagnosis of AIP. Because of the high incidence of malignant neoplasms in AIP patients, it is recommended that male AIP patients over 70 years old who have been diagnosed with AIP within the previous year be examined for malignancy by a combination of gastrointestinal endoscopy, either chest X-ray or CT scan, and abdominal ultrasound.
The authors state that they have no conflicts of interest (COI) to declare.