- (2008) Volume 9, Issue 2
Generoso Uomo, Simona Miraglia
Department of Internal Medicine, Cardarelli Hospital. Naples, Italy
Compartment Syndromes; Infection; Pancreatitis, Acute Necrotizing; Surgical Procedures, Operative
ACS: abdominal compartment syndrome; IAH: intra-abdominal hypertension; SAP: severe acute pancreatitis
Acute pancreatitis may range from mild selflimiting forms to the more severe forms which are affected by high morbidity and mortality [1, 2, 3]. Severe acute pancreatitis (SAP) accounts for 15-30% of all episodes of acute pancreatitis and presents many therapeutic challenges [4, 5, 6]. One important controversial issue concerns the indication and the timing for surgery in these patients. To simplify the question, we have to consider two possible scenarios of SAP: the occurrence of infected pancreatic necrosis, and sterile pancreatic necrosis.
The infection of pancreatic necrosis is associated with a more severe prognosis; immediate surgery (debridement with multiple lavage-drainage techniques) is currently recommended to counteract this important complication [1, 7, 8]. Many international guidelines agree with this indication, but, over the last ten years, some controversy has arisen regarding this topic, especially concerning the timing of the surgical approach. Some reports have shown that early surgical intervention for pancreatic necrosis could result in a worse prognosis in comparison with patients who underwent surgery at a later phase of the disease [9, 10]. Within the subgroup of patients with documented infection of necrosis, a decrease in complications and mortality seems to be related to delaying surgery as long as possible [11, 12, 13]. In addition, some reports surprisingly showed the efficacy of nonsurgical management for infected pancreatic necrosis [14, 15, 16, 17, 18, 19]. Therefore, intensive non-surgical treatment should be considered as an initial, effective treatment modality for patients with SAP and infected necrosis with the aim of delaying (or avoiding in selected cases) surgery in this critically ill group of patients [20]. Certainly, in this situation, we need additional parameters in order to help optimize and standardize our behavior in clinical practice. The second controversial setting regards the best treatment option for patients with SAP and sterile necrosis. Independently from what has been suggested by the international guidelines, wide disagreement exists in clinical practice and the most relevant burden is linked to the clinicians’ expertise or to the attitude of the individual working-care group. Patients with sterile pancreatic necrosis should be managed conservatively with surgery which is usually only required when a large necrotic collection is causing persistent unremitting symptoms [4, 5, 6]. However, many questions arise from this simplification: i) how long should it take to define a “persistent severe” attack of SAP?; ii) how large should the necrotic sterile collection be to be defined as requiring surgery?; iii) how long should the time be to define the condition of “failure to improve” in conservative treatment?. Therefore, for this situation, we surely also need other new parameters aimed at guiding in our decisions in clinical practice a more standardized way. Computed tomography/magnetic resonance features, severity indexes based upon different multiple criteria, and laboratory data (C-reactive protein, interleukins, trypsinogen activation-peptide concentration and other more sophisticated analytes) do not add anything definitive to this context.
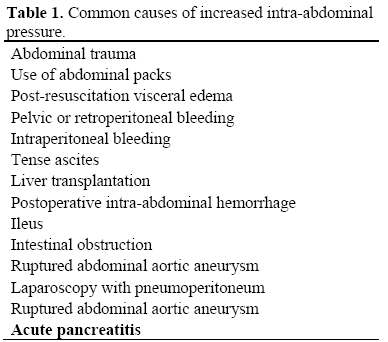
Recently, some interesting articles stressed the clinical relevance of intra-abdominal hypertension in patients with SAP [21, 22, 23, 24, 25]. Intra-abdominal hypertension (IAH) is defined as intra-abdominal pressure greater than 15 mmHg [26] which may occurs in different conditions (Table 1). IAH impairs organ perfusion and leads to organ dysfunction. The combination of IAH with organ failure characterizes the abdominal compartment syndrome (ACS) which has most extensively been described in patients who underwent emergency abdominal surgery or experienced after abdominal trauma [27, 28]. The adverse effects on organ function generally consist of a decrease in cardiac output with hemodynamic instability necessitating inotropic support, oliguria with renal dysfunction, hypoxia, hypercapnia and acidosis [29]. These features usually appear in patients with intra-abdominal pressure greater than 20-25 mmHg (IAH grade III-IV, Table 2). Patients suffering from SAP may develop IAH for several reasons. Pancreatic and retroperitoneal inflammations are the most obvious causes in the early course of the disease. Furthermore, a paralytic ileus and peripancreatic fluid collections with thirdspace loss can also increase IAH. All the same, aggressive fluid resuscitation resulting in generalized and visceral edema can increase the intra-abdominal volume. Some recent studies suggest that IAH is a frequent finding in SAP patients. Pupelis et al. [22] found a relationship between elevated intraabdominal pressure (IAP above 25 mmHg) and persistent subsequent organ dysfunction. Tao et al. [23] described a high incidence of IAH in patients with early SAP, but the lack of a standardized definition of IAH and methodological issues make interpretation of these data difficult. De Waele et al. [24] showed a 51% frequency of IAH in a group of 44 patients with SAP. Analysis of this study revealed that APACHE II and Ranson scores, incidence of organ dysfunction, ICU and total hospital stay were significantly higher in patients with IAH in comparison to those who presented normal intra-abdominal pressure; mortality was also higher but did not reach statistical significance. Very recently, Al-Barhani et al. [25] reported an incidence of 61% of IAH and 56% of ACS in a selected well-studied and monitored group of SAP patients. The authors observed that patients who developed IAH/ACS had a significantly higher organ failure score and greater mortality as compared to those who did not. Percutaneous drainage of fluid collections and decompressive laparotomy with or without laparotomy in patients who fail to respond to percutaneous decompression represent the therapeutic options [28, 30].
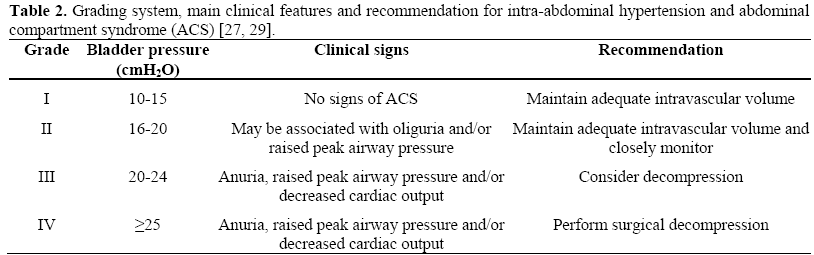
Considering all the above-mentioned data, we can speculate that a rational indication for surgery in SAP should also be evaluated from a “manometric” point of view. In other words, patients with SAP, especially those with extensive sterile necrosis and/or fluid collections and tense abdomen, should be evaluated for the occurrence of IAH/ACS. Careful monitoring is necessary as, in most cases, the development of IAH grade III-IV requires a decompressive procedure.
The authors have no potential conflicts of interest