Khattab Al-Khafaji1, Eyup Ilker Saygili2,3, Tugba Taskin-Tok1,4*, Zafer Cetin5,6, Selin Sayın7, Sinem Ugur7, Merve Goksin Karaaslan8, Oral Cenk Aktas9, Haroon Khan10 and Esra Küpeli Akkol11
1Department of Chemistry, Faculty of Arts and Sciences, Gaziantep University, Turkey
2Department of Medical Biochemistry, School of Medicine, SANKO University, Turkey
3Department of Molecular Medicine, Graduate Institute of Education, SANKO University, Turkey
4Department of Bioinformatics and Computational Biology, Gaziantep University, Turkey
5Department of Medical Biology, School of Medicine, SANKO University, Turkey
6Department of Biological and Biomedical Sciences, Graduate Education Institute, SANKO University, Turkey
7Department of Marine Technologies, Iskenderun Technical University, Turkey
8Tashkent Vocational High School, Selçuk University, Turkey
9Institute of Materials Science, Christian-Albrechts-University, Germany
10Department of Pharmacy, Abdul Wali Khan University Mardan, Pakistan
11Department of Pharmacognosy, Faculty of Pharmacy, Gazi University, Turkey
- *Corresponding Author:
- Tugba Taskin-Tok
Department of Chemistry
Faculty of Arts and Sciences
Gaziantep University, Turkey
Tel: +903122023185
E-mail: ttaskin@gantep.edu.tr
Received Date: October 17, 2020; Accepted Date: January 23, 2021; Published Date: January 30, 2021
Citation: Al-Khafaji K, Saygili EI, Taskin-Tok T, Cetin Z, Sayın S, et al. (2021) Investigation of Promising Antiviral Candidate Molecules based on Algal Phlorotannin for the Prevention of COVID-19 Pandemic by in silico Studies. Biochem Mol Biol Vol.7 No.1:3.
Keywords
ADMET; COVID-19; Spike (S) Protein; Algal phlorotannin; Molecular
docking
Introduction
Coronaviruses are enveloped RNA viruses that cause enteric,
respiratory and central nervous system diseases in various
animals and humans [1]. Coronavirus surface protein spikes (S)
mediate entry into target cells by binding to a cellular receptor
and then fusing the viral envelope with a host cell membrane [2].
SARS-CoV Spike protein (SARS-S) uses Angiotensin Converting
Enzyme 2 (ACE2) as the host cell entry receptor [3,4]. Cleavage of
the S protein by host cell proteases is essential for viral infectivity
and responsible enzymes are potential targets for intervention
[2]. The SARS-S enters angiotensin converting enzyme 2 (ACE2) as the input receptor [3] and uses cellular Transmembrane
Serine Protease 2 (TMPRSS2) for S protein preparation [5]. The
SARS-S/ACE2 interface has been identified at the atomic level
and the effectiveness of ACE2 use has been found to be the main
determinant of SARS-CoV contamination [6].
Outbreaks from viral infectious diseases that have led to mass
deaths throughout history show how they can threaten public
health on a global scale. The current situation clearly shows
that antiviral treatments that are effective against various virus
strains should be developed immediately in the prevention
and treatment of viruses. In the development of antiviral drug
agent, molecules containing innovative functional groups are of
great importance in the structure and effectiveness of drugs.
The targeted properties of functional groups or systems and
their effect on drug composition are very important [7]. At this
point, the use of in silico methods has an important place in the
development of antiviral drug agents’ researches. In predicting
the interactions of bioactive molecules and biological life systems
with each other, the use of in silico methods saves considerable
time, labor and cost.
In recent years, one of the alternative and sustainable ways
of developing effective treatments against the related virus
is the identification of potent agents. In this study, algae; it
is a rich source of effective molecules such as phlorotannins,
polysaccharides, pigments, glycolipids, catechin, terpenoids,
polyhydroxyburates. Algae with their rich functional contents; It
has an important potential with its biological activities such as
anticancer, antimicrobial, anti-inflammatory and antiviral [8].
Phlorotannin is a class of polyphenol compounds produced by
brown seaweed as secondary metabolites and biosynthesized
through the acetate malonate pathway [9,10]. These compounds
have attracted considerable research interest for their broad
health benefits and potential uses in a range of therapeutics
[11,12]. It has demonstrated that phlorotannins can have
anti-diabetic, anti-cancer, anti-oxidation, antibacterial, radio
protective and anti-HIV properties [13,14].
Based on this information, using in silico approaches
within the scope of the study, the inhibitory effect of 11
compounds (Phloroglucinol, Eckol, Fucodiphloroechol-G,
Phlorofucofuroeckol A, 7-Phloroeckol, Dieckol, 6,6'-Bieckol,
Diphloroethohydroxycarmalol, 8,8'-Bieckol, Phlorofucofuroeckol
B, Catechin) on the mechanism of action of SARS-CoV-2 was
investigated. The potent molecules activity has been investigated
and evaluated against the ACE2 and TMPRSS2 proteins to which
SARS-CoV-2 binds.
Materials and Methods
Ligand-protein docking
The data set was composed of 11 compounds (Table 1) which
were obtained from literature [15-22]. These natural compounds
produce from algal organisms that shown antiviral activity were
also remarked in introduction part of the study. Nowadays, remdesivir is the most hopeful SARS-CoV-2 drug, although Food
and Drug Administration (FDA) has also confirmed the utilization of chloroquine and hydroxychloroquine for emergency coronavirus
treatment [23]. The following process, Discovery Studio (DS)
2019 (BIOVIA, 2016) was applied to arrange and to exert the
docking calculations and also to define docking interactions of
the selected compounds- SARS CoV-2-RBD/ACE2 and the selected
compounds- SARS CoV-2-Spike/TMPRSS2 complexes. The crystal
structures of target models, SARS CoV-2-RBD/ACE2 (PDB: 2AJF)
was retrieved from protein data bank [24,25] and SARS CoV-2-
Spike/TMPRSS2 was occurred based on Meng et al. study [26] by
using Homology modelling for docking processes. The ligands, the
selected eleven compounds were sketched and optimized in gas
phase using the CHARMm force field [27] to prepare an ensemble
of docking study with no atomic clashes in their geometries.
| Name |
Herbal name |
Chemical Structure, SMILES |
Molecular Formula |
| 1 |
Phloroglucinol |
Oc1cc(O)cc(O)c1 |
C6 H6 O3 |
| 2 |
Eckol |
Oc1cc(O)cc(Oc2c(O)cc(O)c3Oc4cc(O)cc(O)c4Oc23)c1 |
C18 H12 O9 |
| 3 |
Fucodiphloroechol – G |
Oc1cc(O)c(Oc2cc(O)cc(O)c2Oc3cc(O)cc(O)c3c4c(O)cc(O)cc4O)c(O)c1 |
C24 H18 O12 |
| 4 |
Phlorofucofuroeckol A |
Oc1cc(O)cc(Oc2c(O)cc(O)c3Oc4c(Oc23)c(O)cc5oc6c(Oc7cc(O)cc(O)c7)c(O)cc(O)c6c45)c1 |
C30 H18 O14 |
| 5 |
7-Phloroeckol |
Oc1cc(O)c(Oc2cc(O)c3Oc4c(Oc5cc(O)cc(O)c5)c(O)cc(O)c4Oc3c2)c(O)c1 |
C24 H16 O12 |
| 6 |
Dieckol |
Oc1cc(O)cc(Oc2c(O)cc(O)c3Oc4cc(Oc5c(O)cc(Oc6c(O)cc(O)c7Oc8cc(O)cc(O)c8Oc67)cc5O)cc(O)c4Oc23)c1 |
C36 H22 O18 |
| 7 |
6,6-Bieckol |
Oc1cc(O)cc(Oc2c(O)cc(O)c3Oc4c(Oc23)c(O)cc(O)c4c5c(O)cc(O)c6Oc7c(Oc8cc(O)cc(O)c8)c(O)cc(O)c7Oc56)c1 |
C36 H22 O18 |
| 8 |
Diphloroethohydroxycarmalol |
Oc1cc(O)c(Oc2cc3Oc4c(O)c(Oc5cc(O)cc(O)c5)c(O)c(O)c4Oc3c(O)c2O)c(O)c1 |
C24 H16 O14 |
| 9 |
8,8-Bieckol |
Oc1cc(O)cc(Oc2c(O)cc(O)c3Oc4cc(O)c(c(O)c4Oc23)c5c(O)cc6Oc7c(O)cc(O)c(Oc8cc(O)cc(O)c8)c7Oc6c5O)c1 |
C36 H22 O18 |
| 10 |
Phlorofucofuroeckol B |
Oc1cc(O)cc(Oc2c(O)cc(O)c3Oc4cc5oc6c(Oc7cc(O)cc(O)c7)c(O)cc(O)c6c5c(O)c4Oc23)c1 |
C30 H18 O14 |
| 11 |
Catechin |
O[C@H]1Cc2c(O)cc(O)cc2O[C@@H]1c3ccc(O)c(O)c3 |
C15 H14 O6 |
| *Remdesivir |
Remdesivir |
CCC(CC)COC(=O)[C@H](C)N[P@@](=O)(OC[C@H]1O[C@](C#N)([C@H](O)[C@@H]1O)c2ccc3c(N)ncnn23)Oc4ccccc4 |
C27 H35 N6 O8 P |
| *Chloroquine |
Chloroquine |
CCN(CC)CCC[C@@H](C)Nc1ccnc2cc(Cl)ccc12 |
C18 H26 Cl N3 |
| *Hydroxychloroquine sulfate |
Hydroxychloroquine sulfate |
CCN(CCO)CCC[C@H](C)Nc1ccnc2cc(Cl)ccc12 |
C18 H28 Cl N3 O5 S |
*Remdesivir, chloroquine and hydroxychloroquine are potent drugs against SARS CoV-2 as positive controls.
Table 1: Chemical structures of 11 compounds produce from algal organisms.
In addition, their conformational analyses were investigated
by using DS 2019. On the other hand, both virus models were
prepared using DS tools and minimized until the root mean square
deviation (RMSD) reaches the lower value of 0.05 kcal/mol Å2.
The binding site tool in DS software and the related literatures
information were used to detect binding site of the SARS CoV-2-
RBD/ACE2 and SARS CoV-2-Spike/TMPRSS2 against the selected
eleven natural structures.
Molecular docking is one of the most common procedures for
generating ligand pose inside the pocket and determining the key
residues which interact with ligand. Therefore, docking studies
were executed using the docking software AutodockFR (ADFR)
software [28] with the AutoGridFr (AGFR version 1.0) [29,30]
which is responsible for building configuration file which contains
the data for running controlled flexible docking by detecting
the residues of the complex’s binding site. This enables ligands
reaches buried grooves after running docking calculations by
running of ADFR with presumptive parameters for all complexes
[31]. The docking results for each complex was ranked according
to the binding energy, root mean square deviation (RMSD) and
interaction types.
In silico ADMET prediction
As known that the effectiveness and safety of a potential drug
agent depends essentially on the biotransformations that occur
in the organism. Therefore, drug-likeness properties including
Lipinski [32] and Veber [33] tests for the selected compounds
and remdesivir, chloroquine and hydroxychloroquine sulfate are
effective drugs against SARS CoV-2 as positive controls were used
and filtered by using DS 2019 [34]. In the following step, ADMET
(Absorption, Distribution, Metabolism, Excretion and Toxicity)
prediction was applied for the same compounds with help of
ADMET subprotocol of Discovery Studio 2019 software using the
prediction model by Egan et al. [35,36]. Water solubility (log S),
CaCO2 cell permeability for the prediction of oral drug absorption, Human ether-a-go-go related gene (hERG) inhibition and toxicity
descriptors (AMES toxicity, Hepatotoxicity and Skin sensitization)
were calculated. In summary, it was applied to allow a deeper
insight into applicability of the selected compounds to be safe for
potential drug development against SARS-CoV- 2.
Results
Molecular docking study
For effective docking process, a potential drug agent should
fit the active site of an individual target. That means nonbonding
interactions including hydrogen bond, electrostatic and
hydrophobic have a tremendous impact on docking results. The
binding energies of the molecules shape complementarity are
also an indispensable condition. The key is to define the correct
binding mode with most stable interactions. Binding energy
values of the selected ligands represent their affinity to form
durable interactions inside the pockets of both targets. A low
binding energy value signifies a strong binding and vice versa [37].
Based on these information, out of 11 ligands, only a 33% showed
good binding affinity toward SARS CoV-2-RBD/ACE2 interface,
compared as remdesivir, chloroquine and hydroxychloroquine
sulfate. However, this ratio raised to 42% when 11 ligands docked
to SARS CoV-2-Spike/TMPRSS2 interface as presented in the Table 2. What is intriguing about the data in this table is that
both of Dieckol (6) and Phlorofucofuroeckol B (10) are ranked of
top three against both targets. We attended to explore the top
three compounds and their interactions with each of the selected
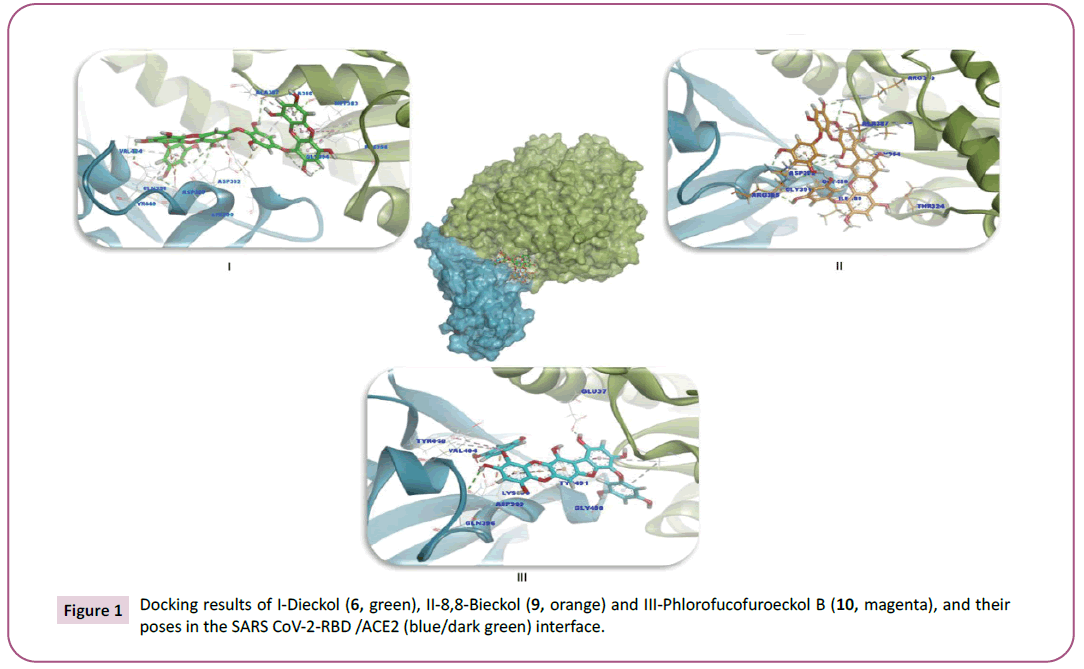
complex. The top one is Dieckol (6) when it binds to SARS CoV-2-
RBD/ACE2. The interactions of Dieckol (6) with the lowest binding affinity (-7.406 kcal/mol) as displayed in Table 3 which forms five
hydrogen bonds with Gly354, Ala386 of ACE2 and six hydrogen
bonds with Lys390, Gln396, Tyr491, Asp393 residues of SARS CoV-
2-RBD protein. Besides hydrogen bond, there are five electrostatic
interactions with Lys390, Asp392 and Asp393 of ACE2 and eight
hydrophobic interactions with four residues of ACE2 protein
(Phe356, Met383, Ala386 and Ala387) and also two residues of
SARS-CoV-2-RBD protein (Tyr440 and Val404) (Figure 1). While
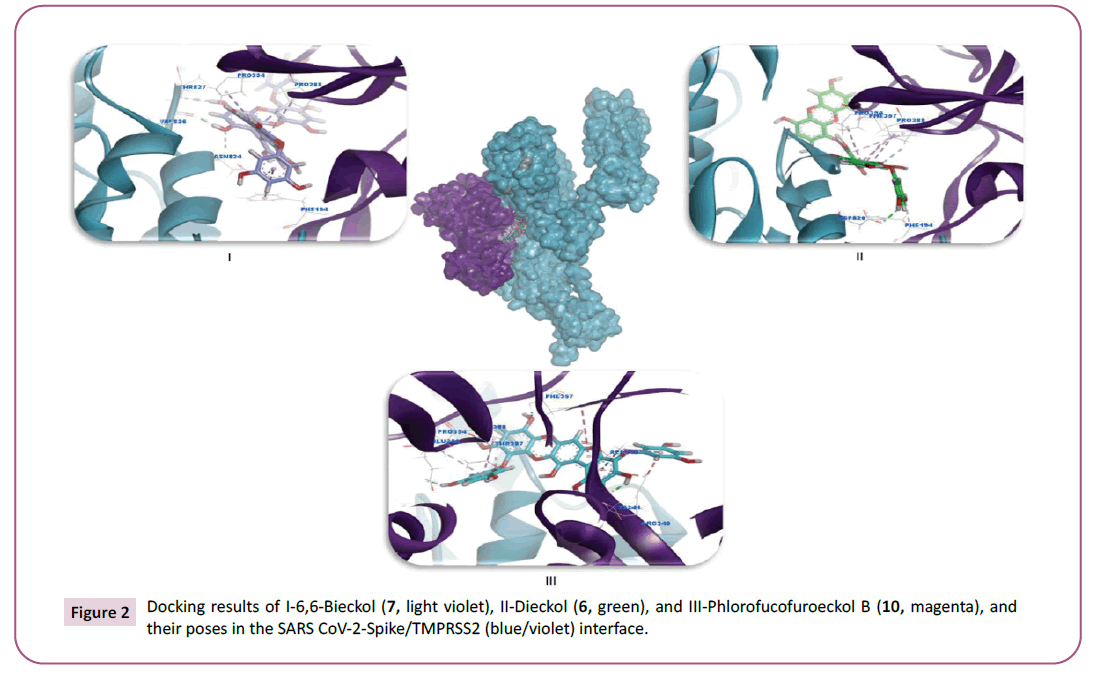
Dieckol comes in second rank of binding affinity (-9.039 kcal/mol)
to bind with SARS CoV-2-Spike/TMPRSS2. Where it formed three
hydrogen bonds and five hydrophobic interactions as shown in Figure 2, where Dieckol (6) interacted in the interface of SARS
CoV-2-Spike/TMPRSS2, through forming two hydrogen bonds
with both Phe194 and Pro288 and five vander Waal interactions
with Pro288, Phe357 and Pro354 of TMPRSS2 protein. Besides,
it forms one hydrogen bond through Asp820 of SARS CoV-2-
Spike. Another significant finding is related to 8,8-Bieckol (9)
which ranked second when it bound to SARS CoV-2-RBD/ACE2
complex with the binding affinity of – 7.253 kcal/mol. First,
8,8-Bieckol (9) interacted with SARS CoV-2-RBD/ACE2 interface
through two conventional hydrogen bonds all of them with ACE2
protein residues (Arg393) and two carbon-hydrogen bonds with
Thr324 and Gly354 as presented in Figure 1. Furthermore, the
residues (Ala386 and Ala387) of ACE2 form three hydrophobic
interactions with 8,8-Bieckol (9). Despite these interactions, SARS
CoV-2-RBD interacted with 8,8-Bieckol (9) through nine hydrogen
bonds (Arg395, Gly490, Asp392, Gly391 and Gly490) and three
electrostatic interactions through Arg395 and Asp392 of SARS
CoV-2-RBD, and also one hydrophobic interaction with Ile489
residue in the related protein, (Figure 1).
| Name |
Donor HB |
Accpt HB |
logS |
Caco-2 |
loghERG |
AMES toxicity |
Hepatotoxicity |
Skin sensitization |
| Range |
2/20 |
2/20 |
-6.5/0.5 |
<25 poor, >500 great |
<-5 |
|
True: Toxic/False: Non-Toxic |
|
| 1 |
3 |
2 |
-0.384 |
269.113 |
-3.257 |
Non-Mutagen |
False |
Strong |
| 2 |
6 |
6 |
-2.619 |
17.844 |
-5.201 |
Non-Mutagen |
False |
Strong |
| 3 |
10 |
9 |
-2.883 |
0.919 |
-6.411 |
Non-Mutagen |
False |
Strong |
| 4 |
9 |
9 |
-4.279 |
1.791 |
-6.85 |
Mutagen |
False |
None |
| 5 |
8 |
8 |
-3.484 |
2.728 |
-6.292 |
Non-Mutagen |
False |
Strong |
| 6 |
11 |
12 |
-5.313 |
0.162 |
-7.929 |
Non-Mutagen |
False |
Strong |
| 7 |
12 |
12 |
-3.89 |
0.344 |
-6.761 |
Non-Mutagen |
False |
Strong |
| 8 |
10 |
10 |
-3.265 |
0.327 |
-6.506 |
Non-Mutagen |
False |
Strong |
| 9 |
12 |
12 |
-3.993 |
0.219 |
-6.805 |
Non-Mutagen |
False |
Strong |
| 10 |
9 |
9 |
-4.325 |
1.539 |
-6.913 |
Mutagen |
False |
None |
| 11 |
5 |
5 |
-2.591 |
57.005 |
-4.784 |
Non-Mutagen |
False |
Strong |
| *Remdesivir |
5 |
17 |
-5.107 |
33.727 |
-6.788 |
Non-Mutagen |
True |
None |
| *Chloroquine |
2 |
6 |
-3.198 |
438.709 |
-5.941 |
Mutagen |
True |
None |
| *Hydroxychloroquine |
1 |
4 |
-3.82 |
1403.192 |
-5.766 |
Mutagen |
True |
None |
Table 2: ADMET analysis of 11 compounds and positive controls* (Remdesivir, chloroquine and hydroxychloroquine sulfate) for SARS CoV-2-RBD/ACE2 and SARS CoV-2-Spike/TMPRSS2).
Figure 1: Docking results of I-Dieckol (6, green), II-8,8-Bieckol (9, orange) and III-Phlorofucofuroeckol B (10, magenta), and their
poses in the SARS CoV-2-RBD /ACE2 (blue/dark green) interface.
Figure 2: Docking results of I-6,6-Bieckol (7, light violet), II-Dieckol (6, green), and III-Phlorofucofuroeckol B (10, magenta), and
their poses in the SARS CoV-2-Spike/TMPRSS2 (blue/violet) interface.
Turning now to another promising compound that is 6,6-Bieckol
(7) which has lowest binding score (-9.177 kcal//mol), with
four hydrogen bonds with when it binds to SARS CoV-2-Spike/
TMPRSS2. Where it forms four hydrogen bonds with SARS CoV-
2 Spike protein residues (Thr827, Val826 and Asn824) and four
hydrophobic interactions with TMPRSS2 residues (Pro288,
Phe194 and Pro354) as shown in Figure 2. On the other hand,
phlorofucofuroeckol B (10) ranked as third when it screened
against SARS CoV-2-RBD/ACE2 and SARS CoV-2-Spike/TMPRSS2
of binding affinity (-6.939 and -8.515 kcal/mol), respectively. This
compound interacted three hydrogen bonds with the residues of
SARS CoV-2-RBD (Lys390, Gln396 and Tyr491) and five electrostatic
interactions with Lys390 and Asp393 residues and also three
hydrophobic interactions with Tyr440, Tyr491 and Val404 of
SARS CoV-2-RBD. In addition, it interacted through forming one
hydrogen bond (Glu37) and three hydrophobic interactions with ACE2 protein residues (Ala386), Figure 1. The compound,
phlorofucofuroeckol B (10) has a good ability to establish four
conventional hydrogen bonds with TMPRSS2 residues (Phe357,
Thr287, Glu289, Cys241) and one carbon-hydrogen bonds
with TMPRSS2 residues (Pro288) as shown in Figure 2. Also, it
interacted through forming two electrostatic interactions with
Arg240 of TMPRSS2 and five hydrophobic interactions with
TMPRSS2 residues (Phe357, Ala243, Pro288, Pro354 and Ala243).
This indicative interaction tells us that phlorofucofuroeckol B (10)
prefers to bind with TMPRSS2 protein.
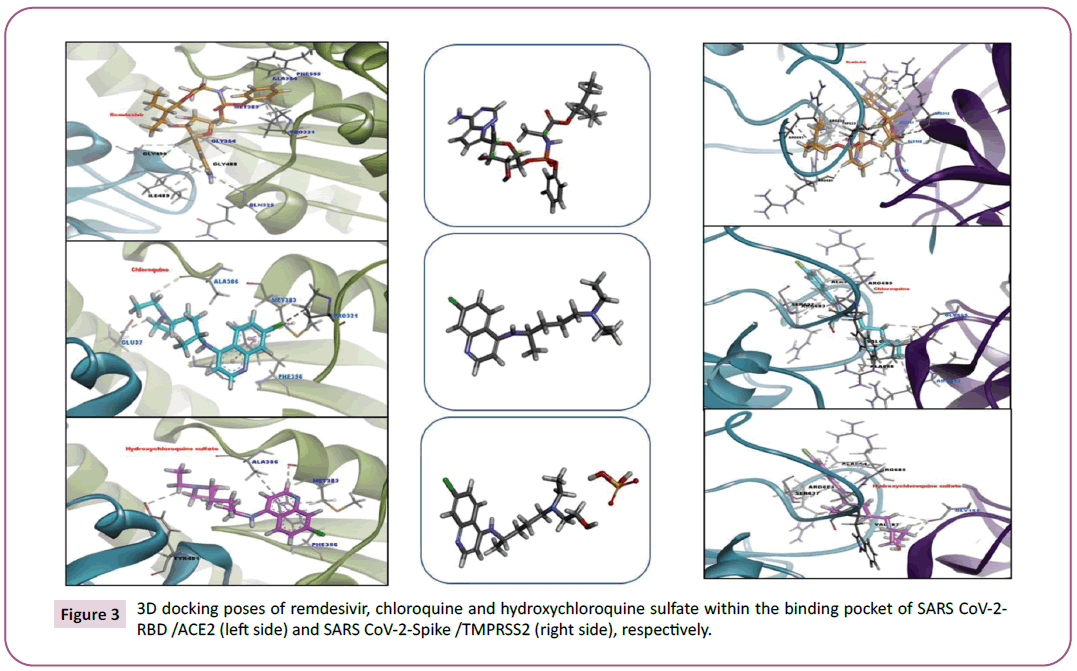
Besides these docking calculations, the same docking processes
were also applied by using three potent drugs against SARS CoV-
2 as three positive controls: remdesivir, is noted for its capacity
to reduce the viral load in the lung tissue of mice infected with
the MERS-CoV virus, improving lung function and damage to
lung tissue [38] and, chloroquine and hydroxychloroquine sulfate are further used anti-malarial drugs recommended by the FDA
against SARS CoV-2 [23]. (Figure 3 and Table 4). Compared
with control compounds and also with the orientation and
conformation of the studied top three compounds at the SARS
CoV-2-RBD/ACE2 and SARS CoV-2-Spike/TMPRSS2 pockets allow
more potent binding (Table 3) and efficient interactions (Figure 1 and Figure 2). In summary, it was revealed that dieckol (6)
and phlorofucofuroeckol B (10) prefer to bind with ACE2 and
TMPRSS2 receptors more than SARS CoV-2 Spike and SARS CoV-
2-RBD proteins. So that the compound (dieckol) can be protector
for the cell receptors (ACE2 and TMPRSS2). Phlorofucofuroeckol
B can also be protector for only TMPRSS2.
Figure 3: 3D docking poses of remdesivir, chloroquine and hydroxychloroquine sulfate within the binding pocket of SARS CoV-2-
RBD /ACE2 (left side) and SARS CoV-2-Spike /TMPRSS2 (right side), respectively.
| Compound Name |
SARS CoV-2-RBD/ACE2 |
SARS CoV-2-Spike/TMPRSS2 |
| Binding energy (kcal/mol) |
RMSD (Å) |
Binding energy (kcal/mol) |
RMSD (Å) |
| 1 |
-4.272 |
0.859 |
-4.952 |
0.474 |
| 2 |
-5.412 |
1.223 |
-7.257 |
1.173 |
| 3 |
-6.039 |
2.041 |
-7.325 |
3.791 |
| 4 |
-6.672 |
2.302 |
-7.942 |
2.125 |
| 5 |
-6.229 |
1.290 |
-8.283 |
1.512 |
| 6 |
-7.406 |
2.021 |
-9.039 |
3.679 |
| 7 |
-6.651 |
1.516 |
-9.177 |
1.058 |
| 8 |
-6.188 |
3.532 |
-8.313 |
3.601 |
| 9 |
-7.253 |
2.786 |
-8.285 |
2.260 |
| 10 |
-6.939 |
1.955 |
-8.515 |
1.801 |
| 11 |
-5.196 |
1.291 |
-6.240 |
1.619 |
| *Remdesivir |
-6.537 |
3.562 |
-8.011 |
2.269 |
| *Chloroquine |
-5.234 |
2.310 |
-5.738 |
1.429 |
| *Hydroxychloroquine sulfate |
-5.185 |
1.873 |
-5.920 |
2.235 |
*Remdesivir, chloroquine and hydroxychloroquine sulfate are potent drugs against SARS CoV-2 as positive controls.
Table 3: Docking Results of 11 compounds toward SARS CoV-2-RBD/ACE2 and SARS CoV-2-Spike/TMPRSS2 complex.
| SARS CoV-2-RBD/ACE2 |
| Interactions-Remdesivir |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| A: GLN325: HN - Remdesivir: N30 |
2.7938 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: GLN325: HN |
Remdesivir: N30 |
| E: GLY490: HN - Remdesivir: N26 |
2.4758 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: GLY490: HN |
Remdesivir: N26 |
| Remdesivir: H47 - A: MET383: O |
2.9175 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: MET383: O |
Remdesivir: H47 |
| A: GLY354: HA1 - Remdesivir: N26 |
2.5265 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: GLY354: HA1 |
Remdesivir: N26 |
| E: GLY488: HA1 - Remdesivir: N26 |
2.6010 |
Hydrogen Bond |
Carbon Hydrogen Bond |
E: GLY488: HA1 |
Remdesivir: N26 |
| E: GLY490: HA1 - Remdesivir: N26 |
3.0246 |
Hydrogen Bond |
Carbon Hydrogen Bond |
E: GLY490: HA1 |
Remdesivir: N26 |
| E: ILE489: HN - Remdesivir |
3.0680 |
Hydrogen Bond |
Pi-Donor Hydrogen Bond |
E: ILE489: HN |
Remdesivir |
| Remdesivir - A: PRO321 |
4.2456 |
Hydrophobic |
Pi-Alkyl |
A: PRO321 |
Remdesivir |
| Remdesivir - A: ALA384 |
4.7492 |
Hydrophobic |
Pi-Alkyl |
A: ALA384 |
Remdesivir |
| Remdesivir - E: ILE489 |
4.7178 |
Hydrophobic |
Pi-Alkyl |
E: ILE489 |
Remdesivir |
| Interactions-Chloroquine |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| Chloroquine: H40 - A: ALA386: O |
2.8499 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: ALA386: O |
Chloroquine: H40 |
| Chloroquine: H42 - A: GLU37: OE1 |
2.8475 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: GLU37: OE1 |
Chloroquine: H42 |
| Chloroquine-A: PHE356 |
5.7629 |
Hydrophobic |
Pi-Pi T-shaped |
A: PHE356 |
Chloroquine |
| Chloroquine- A: PHE356 |
5.1758 |
Hydrophobic |
Pi-Pi T-shaped |
A: PHE356 |
Chloroquine |
| Chloroquine: CL20 - A: PRO321 |
4.0220 |
Hydrophobic |
Alkyl |
A: PRO321 |
Chloroquine: CL20 |
| Chloroquine: CL20 - A: MET383 |
3.5397 |
Hydrophobic |
Alkyl |
A: MET383 |
Chloroquine: CL20 |
| Chloroquine - A: MET383 |
4.7875 |
Hydrophobic |
Pi-Alkyl |
A: MET383 |
Chloroquine |
| Interactions-Hydroxychloroquine sulfate |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| Hydroxychloroquine sulfate: H27 - E: TYR491: OH |
3.0534 |
Hydrogen Bond |
Carbon Hydrogen Bond |
E: TYR491: OH |
Hydroxychloroquine sulfate: H27 |
| Hydroxychloroquine sulfate: H43 - A: MET383: O |
2.6589 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: MET383: O |
Hydroxychloroquine sulfate: H43 |
| Hydroxychloroquine sulfate - A: PHE356 |
5.1956 |
Hydrophobic |
Pi-Pi T-shaped |
A: PHE356 |
Hydroxychloroquine sulfate |
| Hydroxychloroquine sulfate - A: MET383 |
4.9256 |
Hydrophobic |
Pi-Alkyl |
A: MET383 |
Hydroxychloroquine sulfate |
| Hydroxychloroquine sulfate - A: ALA386 |
5.4130 |
Hydrophobic |
Pi-Alkyl |
A: ALA386 |
Hydroxychloroquine sulfate |
| Interactions-Dieckol |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| E: LYS390: HZ2 - Dieckol: O45 |
2.4977 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: LYS390: HZ2 |
Dieckol: O45 |
| E: LYS390: HZ3 - Dieckol: O45 |
2.9174 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: LYS390: HZ3 |
Dieckol: O45 |
| Dieckol: H59 - A: GLY354: O |
2.9561 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: GLY354: O |
Dieckol: H59 |
| Dieckol: H64 - A: ALA386: O |
2.8949 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: ALA386: O |
Dieckol: H64 |
| Dieckol: H70 - E: GLN396: OE1 |
2.8332 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: GLN396: OE1 |
Dieckol: H70 |
| Dieckol: H71 - E: TYR491: OH |
1.9152 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: TYR491: OH |
Dieckol: H71 |
| Dieckol: H75 - E: ASP393: OD2 |
2.7816 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: ASP393: OD2 |
Dieckol: H75 |
| A: GLY354: HA1 - Dieckol: O16 |
2.5775 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: GLY354: HA1 |
Dieckol: O16 |
| A: GLY354: HA1 - Dieckol: O19 |
2.9464 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: GLY354: HA1 |
Dieckol: O19 |
| A: GLY354: HA2 - Dieckol: O19 |
3.0035 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: GLY354: HA2 |
Dieckol: O19 |
| E: LYS390: HZ3 - Dieckol |
2.3468 |
Hydrogen Bond;Electrostatic |
Pi-Cation;Pi-Donor Hydrogen Bond |
E: LYS390: HZ3 |
Dieckol |
| E: ASP392: OD1 - Dieckol |
4.4474 |
Electrostatic |
Pi-Anion |
E: ASP392: OD1 |
Dieckol |
| E: ASP392: OD2 - Dieckol |
3.3188 |
Electrostatic |
Pi-Anion |
E: ASP392: OD2 |
Dieckol |
| E: ASP393: OD1 - Dieckol |
4.5729 |
Electrostatic |
Pi-Anion |
E: ASP393: OD1 |
Dieckol |
| E: ASP393: OD1 - Dieckol |
3.9606 |
Electrostatic |
Pi-Anion |
E: ASP393: OD1 |
Dieckol |
| E: TYR440 - Dieckol |
5.2778 |
Hydrophobic |
Pi-Pi Stacked |
E: TYR440 |
Dieckol |
| A: PHE356 - Dieckol |
5.8437 |
Hydrophobic |
Pi-Pi T-shaped |
A: PHE356 |
Dieckol |
| A: PHE356 - Dieckol |
5.2454 |
Hydrophobic |
Pi-Pi T-shaped |
A: PHE356 |
Dieckol |
| Dieckol - A: MET383 |
5.1850 |
Hydrophobic |
Pi-Alkyl |
A: MET383 |
Dieckol |
| Dieckol - A: MET383 |
5.3465 |
Hydrophobic |
Pi-Alkyl |
A: MET383 |
Dieckol |
| Dieckol - A: ALA386 |
4.6125 |
Hydrophobic |
Pi-Alkyl |
A: ALA386 |
Dieckol |
| Dieckol - A: ALA386 |
4.5717 |
Hydrophobic |
Pi-Alkyl |
A: ALA386 |
Dieckol |
| Dieckol - A: ALA387 |
5.4374 |
Hydrophobic |
Pi-Alkyl |
A: ALA387 |
Dieckol |
| Dieckol - A: ALA386 |
4.9676 |
Hydrophobic |
Pi-Alkyl |
A: ALA386 |
Dieckol |
| Dieckol - E: VAL404 |
4.9313 |
Hydrophobic |
Pi-Alkyl |
E: VAL404 |
Dieckol |
| Interactions-8,8-Bieckol |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| A: ARG393: HH21 - 8,8-Bieckol: O24 |
2.9012 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: ARG393: HH21 |
8,8-Bieckol: O24 |
| A: ARG393: HH21 - 8,8-Bieckol: O34 |
2.2271 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: ARG393: HH21 |
8,8-Bieckol: O34 |
| E: ARG395: HE - 8,8-Bieckol: O45 |
2.4215 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: ARG395: HE |
8,8-Bieckol: O45 |
| E: GLY490: HN - 8,8-Bieckol: O3 |
2.2512 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: GLY490: HN |
8,8-Bieckol: O3 |
| 8,8-Bieckol: H57 - E: ASP392: OD1 |
2.6917 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: ASP392: OD1 |
8,8-Bieckol: H57 |
| 8,8-Bieckol: H66 - E: ASP392: OD2 |
2.6695 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: ASP392: OD2 |
8,8-Bieckol: H66 |
| 8,8-Bieckol: H70 - E: ASP392: O |
2.2937 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: ASP392: O |
8,8-Bieckol: H70 |
| 8,8-Bieckol: H75 - E: ASP392: OD2 |
2.4938 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: ASP392: OD2 |
8,8-Bieckol: H75 |
| 8,8-Bieckol: H76 - E: GLY391: O |
2.6050 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: GLY391: O |
8,8-Bieckol: H76 |
| A: THR324: HB - 8,8-Bieckol: O17 |
2.7874 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: THR324: HB |
8,8-Bieckol: O17 |
| A: GLY354: HA2 - 8,8-Bieckol: O35 |
2.6753 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: GLY354: HA2 |
8,8-Bieckol: O35 |
| E: GLY490: HA1 - 8,8-Bieckol: O3 |
2.9183 |
Hydrogen Bond |
Carbon Hydrogen Bond |
E: GLY490: HA1 |
8,8-Bieckol: O3 |
| E: GLY490: HA1 - 8,8-Bieckol: O15 |
2.5716 |
Hydrogen Bond |
Carbon Hydrogen Bond |
E: GLY490: HA1 |
8,8-Bieckol: O15 |
| E: ARG395: NH2 - 8,8-Bieckol |
4.0496 |
Electrostatic |
Pi-Cation |
E: ARG395: NH2 |
8,8-Bieckol |
| E: ASP392: OD1 - 8,8-Bieckol |
3.7168 |
Electrostatic |
Pi-Anion |
E: ASP392: OD1 |
8,8-Bieckol |
| E: ASP392: OD2 - 8,8-Bieckol |
3.3036 |
Electrostatic |
Pi-Anion |
E: ASP392: OD2 |
8,8-Bieckol |
| A: ALA387: HA - 8,8-Bieckol |
2.6522 |
Hydrophobic |
Pi-Sigma |
A: ALA387: HA |
8,8-Bieckol |
| 8,8-Bieckol - E: ILE489 |
4.7007 |
Hydrophobic |
Pi-Alkyl |
E: ILE489 |
8,8-Bieckol |
| 8,8-Bieckol - A: ALA386 |
4.3628 |
Hydrophobic |
Pi-Alkyl |
A: ALA386 |
8,8-Bieckol |
| 8,8-Bieckol - A: ALA387 |
4.7288 |
Hydrophobic |
Pi-Alkyl |
A: ALA387 |
8,8-Bieckol |
| Interactions-Phlorofucofuroeckol B |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| Phlorofucofuroeckol B: H58 - A: GLU37: OE1 |
2.0637 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: GLU37: OE1 |
Phlorofucofuroeckol B: H58 |
| Phlorofucofuroeckol B: H60 - E: GLN396: OE1 |
2.7084 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: GLN396: OE1 |
Phlorofucofuroeckol B: H60 |
| Phlorofucofuroeckol B: H62 - E: TYR491: OH |
2.6045 |
Hydrogen Bond |
Conventional Hydrogen Bond |
E: TYR491: OH |
Phlorofucofuroeckol B: H62 |
| E: LYS390: NZ - Phlorofucofuroeckol B |
4.9696 |
Electrostatic |
Pi-Cation |
E: LYS390: NZ |
Phlorofucofuroeckol B |
| E: LYS390: NZ - Phlorofucofuroeckol B |
4.6371 |
Electrostatic |
Pi-Cation |
E: LYS390: NZ |
Phlorofucofuroeckol B |
| E: LYS390: HZ3 - Phlorofucofuroeckol B |
2.6050 |
Hydrogen Bond;Electrostatic |
Pi-Cation;Pi-Donor Hydrogen Bond |
E: LYS390: HZ3 |
Phlorofucofuroeckol B |
| E: ASP393: OD1 - Phlorofucofuroeckol B |
4.0933 |
Electrostatic |
Pi-Anion |
E: ASP393: OD1 |
Phlorofucofuroeckol B |
| E: ASP393: OD1 - Phlorofucofuroeckol B |
4.6346 |
Electrostatic |
Pi-Anion |
E: ASP393: OD1 |
Phlorofucofuroeckol B |
| E: TYR440 - Phlorofucofuroeckol B |
5.2464 |
Hydrophobic |
Pi-Pi Stacked |
E: TYR440 |
Phlorofucofuroeckol B |
| E: TYR491 - Phlorofucofuroeckol B |
5.2086 |
Hydrophobic |
Pi-Pi T-shaped |
E: TYR491 |
Phlorofucofuroeckol B |
| Phlorofucofuroeckol B - A: ALA386 |
5.2691 |
Hydrophobic |
Pi-Alkyl |
A: ALA386 |
Phlorofucofuroeckol B |
| Phlorofucofuroeckol B - E: VAL404 |
4.6856 |
Hydrophobic |
Pi-Alkyl |
E: VAL404 |
Phlorofucofuroeckol B |
| Phlorofucofuroeckol B - A: ALA386 |
5.1446 |
Hydrophobic |
Pi-Alkyl |
A: ALA386 |
Phlorofucofuroeckol B |
| SARS CoV-2 Spike/TMPRSS 2 |
| Interactions-Remdesivir |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| Remdesivir: H47 - B: GLY165: O |
2.8345 |
Hydrogen Bond |
Conventional Hydrogen Bond |
B: GLY165: O |
Remdesivir: H47 |
| Remdesivir: H56 - A: VAL687: O |
2.5341 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: VAL687: O |
Remdesivir: H56 |
| Remdesivir: H57 - B: ARG313: O |
2.8787 |
Hydrogen Bond |
Conventional Hydrogen Bond |
B: ARG313: O |
Remdesivir: H57 |
| A: TRP633: HE1 - Remdesivir: N30 |
2.8480 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: TRP633: HE1 |
Remdesivir: N30 |
| B: ARG313: H - Remdesivir: N26 |
2.4144 |
Hydrogen Bond |
Conventional Hydrogen Bond |
B: ARG313: H |
Remdesivir: N26 |
| B: ARG313: HH11 - Remdesivir: N26 |
3.0912 |
Hydrogen Bond |
Conventional Hydrogen Bond |
B: ARG313: HH11 |
Remdesivir: N26 |
| Remdesivir: H43 - A: ARG685: O |
2.9438 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: ARG685: O |
Remdesivir: H43 |
| A: ARG634: HD3 - Remdesivir: O8 |
2.4125 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: ARG634: HD3 |
Remdesivir: O8 |
| B: ALA312: HA - Remdesivir: O24 |
2.9702 |
Hydrogen Bond |
Carbon Hydrogen Bond |
B: ALA312: HA |
Remdesivir: O24 |
| B: ARG167: NH2 - Remdesivir |
4.0186 |
Electrostatic |
Pi-Cation |
B: ARG167: NH2 |
Remdesivir |
| B: ARG167: NH2 - Remdesivir |
4.1807 |
Electrostatic |
Pi-Cation |
B: ARG167: NH2 |
Remdesivir |
| B: ARG313: NH1 - Remdesivir |
4.6223 |
Electrostatic |
Pi-Cation |
B: ARG313: NH1 |
Remdesivir |
| A: ARG634: H - Remdesivir |
2.6939 |
Hydrogen Bond |
Pi-Donor Hydrogen Bond |
A: ARG634: H |
Remdesivir |
| B: GLY166: O - Remdesivir |
2.7178 |
Other |
Pi-Lone Pair |
B: GLY166: O |
Remdesivir |
| Remdesivir: C40 - A: ARG683 |
4.2866 |
Hydrophobic |
Alkyl |
A: ARG683 |
Remdesivir: C40 |
| Remdesivir: C42 - A: ARG634 |
4.5767 |
Hydrophobic |
Alkyl |
A: ARG634 |
Remdesivir: C42 |
| Remdesivir- A: ARG634 |
4.3767 |
Hydrophobic |
Pi-Alkyl |
A: ARG634 |
Remdesivir |
| Remdesivir- B: ARG167 |
4.0580 |
Hydrophobic |
Pi-Alkyl |
B: ARG167 |
Remdesivir |
| Remdesivir- B: ARG167 |
3.7030 |
Hydrophobic |
Pi-Alkyl |
B: ARG167 |
Remdesivir |
| Interactions-Chloroquine |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| A: ALA684: H - Chloroquine: N13 |
2.26467 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: ALA684: H |
Chloroquine: N13 |
| Chloroquine: H36 - B: GLY165: O |
2.8385 |
Hydrogen Bond |
Carbon Hydrogen Bond |
B: GLY165: O |
Chloroquine: H36 |
| Chloroquine: H39 - B: GLY165: O |
3.08589 |
Hydrogen Bond |
Carbon Hydrogen Bond |
B: GLY165: O |
Chloroquine: H39 |
| Chloroquine: H41 - A: VAL687: O |
2.71398 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: VAL687: O |
Chloroquine: H41 |
| Chloroquine: H42 - B: GLY165: O |
2.44242 |
Hydrogen Bond |
Carbon Hydrogen Bond |
B: GLY165: O |
Chloroquine: H42 |
| A: SER637: HG - : Chloroquine |
2.80972 |
Hydrogen Bond |
Pi-Donor Hydrogen Bond |
A: SER637: HG |
Chloroquine |
| Chloroquine: C22 - A: ALA688 |
3.78942 |
Hydrophobic |
Alkyl |
A: ALA688 |
Chloroquine: C22 |
| Chloroquine: C22 - B: ARG313 |
4.43767 |
Hydrophobic |
Alkyl |
B: ARG313 |
Chloroquine: C22 |
| Chloroquine - A: ARG683 |
4.80707 |
Hydrophobic |
Pi-Alkyl |
A: ARG683 |
Chloroquine |
| Chloroquine - A: ARG685 |
4.25457 |
Hydrophobic |
Pi-Alkyl |
A: ARG685 |
Chloroquine |
| Chloroquine - A: ARG685 |
3.99115 |
Hydrophobic |
Pi-Alkyl |
A: ARG685 |
Chloroquine |
| Interactions-Hydroxychloroquine sulfate |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| A: ALA684: H - Hydroxychloroquine sulfate: N17 |
2.27755 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: ALA684: H |
Hydroxychloroquine sulfate: N17 |
| Hydroxychloroquine sulfate: H28 - B: GLY165: O |
2.37459 |
Hydrogen Bond |
Carbon Hydrogen Bond |
B: GLY165: O |
Hydroxychloroquine sulfate: H28 |
| Hydroxychloroquine sulfate: H29 - B: GLY165: O |
2.80296 |
Hydrogen Bond |
Carbon Hydrogen Bond |
B: GLY165: O |
Hydroxychloroquine sulfate: H29 |
| Hydroxychloroquine sulfate: H45 - B: GLY165: O |
2.78093 |
Hydrogen Bond |
Carbon Hydrogen Bond |
B: GLY165: O |
Hydroxychloroquine sulfate: H45 |
| Hydroxychloroquine sulfate: H46 - B: GLY165: O |
2.57049 |
Hydrogen Bond |
Carbon Hydrogen Bond |
B: GLY165: O |
Hydroxychloroquine sulfate: H46 |
| Hydroxychloroquine sulfate: H47 - A: VAL687: O |
2.46144 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: VAL687: O |
Hydroxychloroquine sulfate: H47 |
| Hydroxychloroquine sulfate: H48 - A: VAL687: O |
2.7889 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: VAL687: O |
Hydroxychloroquine sulfate: H48 |
| A: SER637: HG - Hydroxychloroquine sulfate |
2.78346 |
Hydrogen Bond |
Pi-Donor Hydrogen Bond |
A: SER637: HG |
Hydroxychloroquine sulfate |
| Hydroxychloroquine sulfate - A: ARG683 |
4.75047 |
Hydrophobic |
Pi-Alkyl |
A: ARG683 |
Hydroxychloroquine sulfate |
| Hydroxychloroquine sulfate - A: ARG685 |
4.28571 |
Hydrophobic |
Pi-Alkyl |
A: ARG685 |
Hydroxychloroquine sulfate |
| Hydroxychloroquine sulfate - A: ALA684 |
5.24405 |
Hydrophobic |
Pi-Alkyl |
A: ALA684 |
Hydroxychloroquine sulfate |
| Hydroxychloroquine sulfate - A: ARG685 |
3.95296 |
Hydrophobic |
Pi-Alkyl |
A: ARG685 |
Hydroxychloroquine sulfate |
| Interactions-6,6-Bieckol |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| 6,6-Bieckol: H65 - B: THR827: OG1 |
2.4295 |
Hydrogen Bond |
Conventional Hydrogen Bond |
B: THR827: OG1 |
6,6-Bieckol: H65 |
| 6,6-Bieckol: H66 - B: VAL826: O |
1.8942 |
Hydrogen Bond |
Conventional Hydrogen Bond |
B: VAL826: O |
6,6-Bieckol: H66 |
| B: ASN824: HA - 6,6-Bieckol: O36 |
2.8434 |
Hydrogen Bond |
Carbon Hydrogen Bond |
B: ASN824: HA |
6,6-Bieckol: O36 |
| B: ASN824: HD22 - 6,6-Bieckol |
2.6394 |
Hydrogen Bond |
Pi-Donor Hydrogen Bond |
B: ASN824: HD22 |
6,6-Bieckol |
| A: PRO288: HG3 - 6,6-Bieckol |
2.4698 |
Hydrophobic |
Pi-Sigma |
A: PRO288: HG3 |
6,6-Bieckol |
| A: PHE194 - 6,6-Bieckol |
5.1410 |
Hydrophobic |
Pi-Pi T-shaped |
A: PHE194 |
6,6-Bieckol |
| 6,6-Bieckol - A: PRO288 |
4.9211 |
Hydrophobic |
Pi-Alkyl |
A: PRO288 |
6,6-Bieckol |
| 6,6-Bieckol - A: PRO354 |
5.4241 |
Hydrophobic |
Pi-Alkyl |
A: PRO354 |
6,6-Bieckol |
| Interactions-Dieckol |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| Dieckol: H75 - B: ASP820: OD2 |
2.3269 |
Hydrogen Bond |
Conventional Hydrogen Bond |
B: ASP820: OD2 |
Dieckol: H75 |
| A: PHE194: HA - Dieckol: O54 |
2.8352 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: PHE194: HA |
Dieckol: O54 |
| A: PRO288: HD2 - Dieckol: O34 |
2.7823 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: PRO288: HD2 |
Dieckol: O34 |
| A: PRO288: HG3 - Dieckol |
2.8002 |
Hydrophobic |
Pi-Sigma |
A: PRO288: HG3 |
Dieckol |
| A: PHE357 - Dieckol |
5.3177 |
Hydrophobic |
Pi-Pi T-shaped |
A: PHE357 |
Dieckol |
| Dieckol - A: PRO288 |
4.7037 |
Hydrophobic |
Pi-Alkyl |
A: PRO288 |
Dieckol |
| Dieckol - A: PRO288 |
5.1331 |
Hydrophobic |
Pi-Alkyl |
A: PRO288 |
Dieckol |
| Dieckol - A: PRO354 |
4.5933 |
Hydrophobic |
Pi-Alkyl |
A: PRO354 |
Dieckol |
| Interactions-Phlorofucofuroeckol B |
Distance Å |
Bonding |
Bonding Types |
Target |
Ligand |
| A: PHE357: H - Phlorofucofuroeckol B: O38 |
2.3777 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: PHE357: H |
Phlorofucofuroeckol B: O38 |
| Phlorofucofuroeckol B: H56 - A: THR287: OG1 |
2.8288 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: THR287: OG1 |
Phlorofucofuroeckol B: H56 |
| Phlorofucofuroeckol B: H57 - A: GLU289: OE2 |
2.0276 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: GLU289: OE2 |
Phlorofucofuroeckol B: H57 |
| Phlorofucofuroeckol B: H59 - A: CYS241: O |
2.0033 |
Hydrogen Bond |
Conventional Hydrogen Bond |
A: CYS241: O |
Phlorofucofuroeckol B: H59 |
| A: PRO288: HD2 - Phlorofucofuroeckol B: O33 |
2.4845 |
Hydrogen Bond |
Carbon Hydrogen Bond |
A: PRO288: HD2 |
Phlorofucofuroeckol B: O33 |
| A: ARG240: NH1 - Phlorofucofuroeckol B |
3.1654 |
Electrostatic |
Pi-Cation |
A: ARG240: NH1 |
Phlorofucofuroeckol B |
| A: ARG240: NH1 - Phlorofucofuroeckol B |
4.0653 |
Electrostatic |
Pi-Cation |
A: ARG240: NH1 |
Phlorofucofuroeckol B |
| Phlorofucofuroeckol B - A: PHE357 |
5.5184 |
Hydrophobic |
Pi-Pi T-shaped |
A: PHE357 |
Phlorofucofuroeckol B |
| Phlorofucofuroeckol B - A: ALA243 |
5.3830 |
Hydrophobic |
Pi-Alkyl |
A: ALA243 |
Phlorofucofuroeckol B |
| Phlorofucofuroeckol B - A: PRO288 |
4.7384 |
Hydrophobic |
Pi-Alkyl |
A: PRO288 |
Phlorofucofuroeckol B |
| Phlorofucofuroeckol B - A: PRO354 |
4.5468 |
Hydrophobic |
Pi-Alkyl |
A: PRO354 |
Phlorofucofuroeckol B |
| Phlorofucofuroeckol B - A: ALA243 |
4.5363 |
Hydrophobic |
Pi-Alkyl |
A: ALA243 |
Phlorofucofuroeckol B |
Table 4: Interactions types and distances of three positive controls (Remdesivir, chloroquine and hydroxychloroquine sulfate) and the three
better compounds [Diekcol (6), 8,8-Bieckol (9) and Phlorofucofuroeckol B (10)] with SARS CoV-2-RBD/ACE2 and [6,6-Bieckol (7), Diekcol (6) and
Phlorofucofuroeckol B (10)] with SARS CoV-2-Spike/TMPRSS2, respectively.
In silico ADMET analysis
ADMET properties were assessed by ADMET subprotocol of
Discovery Studio 2019 software. It compiles pharmacokinetic
properties for selected ligands along with control compounds
(remdesivir, chloroquine and hydroxychloroquine sulfate) in Table 2. Basically, the poor solubility associates with poor
absorption. So the water solubility (log S) of a compound
significantly influences its absorption features. The predicted
log S values of all top four compounds [dieckol (6), 6,6-Bieckol
(7), 8,8-Bieckol (9) and phlorofucofuroeckol B (10)] were within
the tolerable limit. Donor and acceptor of hydrogen bonding
are essentials of Lipinski rule and all the ligands were displayed
within the acceptable range of drug-likeliness. CaCO2 intestinal
cell line permeability is measured in nm/sec and is meaningful for
intestinal absorption. Its value was lower than the limited value
for among the top four tested ligands. The log hERG (log IC50) values for dieckol (6), phlorofucofuroeckol B (10), 8,8-Bieckol (9)
and 6,6-Bieckol (7) display the finest results among eleven ligands.
The negative value of log hERG shows that the lower the value
of log hERG, the lesser is the blockage of K+ ion channels [37].
The results of in silico ADMET studies implied that dieckol (6) and
phlorofucofuroeckol B (10) exhibited fine pharmacokinetic profile.
Further, the predicted toxicity data (AMES toxicity, hepatotoxicity,
skin sensitization values) reveal that the lead compounds have no
toxicity and present demanded range. Henceforth they could be
foreseen as safe for drug development against human epidemics
of SARS-CoV-2.
Discussion
The mechanisms of antiviral actions of algal bioactive compounds
include direct virucidal action, inhibition of viral attachment to
host cells, inhibition of virus internalization and uncoating in the
target cell, inhibition of viral transcription and replication and
improvement of antiviral immune responses in host cells [39].
Several reports have done that the routes of SARS CoV-2
protein targets, structures and models (Main Protease, Papainlike
protease, Spike RBD, Spike monomer and trimer etc)
administration can affect the nature of the treatment toward
SARS CoV-2. In the meantime, it is present different ligands
having antibody, peptide and small molecules are used to prevent
or stop the activities of the disease. The all documents such as
targets, structure, models, therapeutics... etc. are present in
website, data hub or server systems [40-43].
For example, using the homology modeling models of the Spike
glycoprotein and SARS CoV-2 protease 3CLPRO have developed
and docking analysis were performed by Hall and Ji 2020, utilizing
previously known approved compounds. They suggested several
potent inhibitors on the 3CLPRO main proteinase activity including;
Zanamivir approved for the treatment of influenza A and B
viruses, Indinavir and Saquinavir for treatment of HIV, Remdesivir
at experimental stages that has shown clinical activity against
the SARS-coronavirus, Ebola virus, and possibly the SARS CoV-2,
Flavin Adenine Dinucleotide (FAD) and Coenzyme A [44]. It is also
reported that, the aflavin was able to dock in the catalytic pocket
near the active site of RdRp in SARS CoV‐2, SARS CoV, and MERS
CoV in the two different molecular docking methods [45].
Besides these, the study [46] estimates antagonists of SARS
CoV-2 Mpro, SARS CoV-3CLpro, ACE2 Receptor and NSP12 RNA
Polymerase against COVID-19, based on already approved 28
drugs, using last disease mechanisms discoveries. Further,
it exhibited that hydroxychloroquine, chloroquine were not
showed effective, as monotherapies, against COVID-19 or lung
cell receptors. Herein, this fact was once again revealed at the
molecular level, using silico methods, and the results were
validated that chloroquine is not a suitable and effective drug for
the treatment of COVID-19 in this article.
Based on these results, molecular docking for our study was
performed to explain the effect of the top three molecules
amongst the selected 11 compounds against both targets;SARS
CoV-2-RBD/ACE2 and SARS CoV-2-Spike/TMPRSS2. These targets
play important roles in prevent and transmission pathways of the
related virus and considered as therapeutic targets for disease
treatment. Dieckol (6) efficiently docked to the hydrophobic
groove of SARS CoV-2-RBD/ACE2. Following, 8,8-Bieckol (9)
and Phlorofucofuroeckol B (10) interact with the same target.
Dieckol (6) compound has similar behavior as 8,8-Bieckol (9).
Furthermore, these compounds display good activity with
SARS CoV-2-RBD/ACE2 due to hydroxyl groups in their frame
structures. Dieckol and diphlorethohydroxycarmalol phlorotanins
isolated from Ecklonia cava Kjelman strongly inhibited HIV-1
Reverse Transcriptase (RT) activity and moderately inhibited HIV-
1 protease activity. Dieckol inhibited the syncytium formation and
penetration of HIV into cells, viral replication, and virus induced
lytic effects [47].
For another target, (SARS CoV-2-Spike/TMPRSS2) 6,6-Bieckol (7)
bounds in the position of two proteins interface with the lowest binding energy (-9.177 kcal//mol) in other compounds. In the
meantime, among the polyphenolic compounds 6,6-Bieckol
isolated from Ecklonia cava was found to be inhibitory effect on
HIV-1 induced syncytia formation, cell–virus and cell–cell fusion,
viral entry, HIV-1 RT enzyme activity and cytopathic effects of
HIV-1 in a dose-dependent manner [48]. Second one is Diekcol
(6). Dieckol, eckol, 7-phloroeckol, fucodiphloroethol G and
phlorofucofuroeckol phlorotannins exhibited inhibitory effect on
SARS CoV 3CLpro activity in a dose-dependent fashion. Among
these compounds Dieckol was found to be the most efficient
molecule on inhibiting cleavage activity of the 3CLpro enzyme.
Docking experiments also supported the important inhibitory
effect of Dieckol on SARS CoV 3CLpro enzyme [49]. The last, it
shows third good binding affinity value of Phlorofucofuroeckol
B (10) toward the target. Interestingly, Kwon HJ et al. reported
that, Phlorofucofuroeckol have inhibited Porcine Epidemic
Diarrhea Virus PEDV which is belonging to Coronaviridae family
of viruses through inhibiting its attachment to the target cell.
They also showed that Dieckol, 7-Phloroeckol and Eckol was also
had inhibitory effect on virus and target cell attachment. Dieckol,
Eckol and Phlorofucofuroeckol displayed strong inhibition of
hemaglutination which have been completely blocking virus
attachment at intestinal enterocytes. This antiviral activity
attributed to a strong interaction with S protein on the outer
surface of PEDV which results in restricts the viral adsorption.
Dieckol and Phlorofucofuroeckol were found to be have stronger
inhibitory effects on the late stage viral replication [50]. However,
to our knowledge there is no publication on in silico analysis for
algal phenolic compounds’ binding affinity at ACE2 and TMPRSS2
receptors SARS-COV-2 binding surfaces.
Conclusion
In this study showed that an in silico model of algal molecules
interactivity on SARS CoV-2-RBD/ACE2 and SARS CoV-2-Spike/
TMPRSS2 receptor. In general, computational observations
suggests that the hydroxyl group of the related compounds,
which are largely responsible for antiviral activities, is a good
evidence to refute the existence of current belief. However, to
date, no studies have shown an association of the inhibitory
effect of these molecules with inflammation of SARS-CoV-2. In
summary, this research, based on a broad theoretical approach,
can be a guide for future research to learn about molecules
selected from algae in the treatment or prevention of SARS CoV-2. Pre-clinic studies could be promise as candidate clinical
potential in these molecules over SARS – CoV-2 inflammation or
different pandemics in future.
Declaration of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could
be construed as a potential conflict of interest.
Data Availability Statement
The datasets generated for this study are available on request to
the corresponding authors.
References
- Holmes EC (2003) Error thresholds and the constraints to RNA virus evolution. Trends Microbiol 11: 543-546.
- Gallagher TM, Buchmeier MJ (2001) Coronavirus Spike Proteins in Viral Entry and Pathogenesis. Virology 279: 371–374.
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, et al. (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454.
- Wang T, Jónsdóttir R, Liu H, Gu L, Kristinsson HG, et al. (2012) Antioxidant Capacities of Phlorotannins Extracted from the Brown Algae Fucus vesiculosus. J Agric Food Chem 60: 5874–5883.
- Glowacka I, Bertram S, Muller MA, Allen P, Soilleux E, et al. (2011) Evidence that TMPRSS2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response. J Virol 85: 4122–4134.
- Li F, Li W, Farzan M, Harrison SC (2005) Structure of SARS Coronavirus Spike Receptor-Binding Domain Complexed with Receptor. Science 309: 1864–1868.
- Röhrig B, Prel JB, Wachtlin D, Kwiecien R, Blettner M (2010) Sample Size Calculation in Clinical Trials. Deutsches Aerzteblatt Online.
- Pérez M, Falqué E, Domínguez H (2016) Antimicrobial Action of Compounds from Marine Seaweed. Mar Drugs 14: 52.
- Leyton A, Pezoa-Conte R, Barriga A, Buschmann AH, Maki-Arvela P, et al. (2016) Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res 16: 201–208.
- Meslet-Cladiere L, Delage L, Leroux CJ, Goulitquer S, Leblanc C, et al. (2013) Structure/Function Analysis of a Type III Polyketide Synthase in the Brown Alga Ectocarpus siliculosus Reveals a Biochemical Pathway in Phlorotannin Monomer Biosynthesis. The Plant Cell 25: 3089–103.
- Zubia M, Fabre MS, Kerjean V, Lann KL, Stiger-Pouvreau V, et al. (2009) Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem 116: 693–701.
- Montero L, Sánchez-Camargo AP, García-Cañas V, Tanniou A, Stiger-Pouvreau V, et al. (2016) Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J Chromatogr A 1428: 115–125.
- Gupta S, Abu-Ghannam N (2011) Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci Technol 22: 315–326.
- Li YX, Wijesekara I, Li Y, Kim SK (2011) Phlorotannins as bioactive agents from brown algae. Process Biochem 46: 2219-2224.
- Ancheeva E, El-Neketi M, Daletos G, Ebrahim W, Song W, et al. (2018) Anti-infective Compounds from Marine Organisms. In P. H. Rampelotto & A. Trincone (Edn), Cham: Springer International Publishing. Grand Challenges in Marine Biotechnology 97-155.
- Arisawa M, Fujita A, Hayashi T, Hayashi K, Ochiai H, et al. (1990) Cytotoxic and Antiherpetic Activity of Phloroglucinol Derivatives from Mallotus japonicus (Euphorbiaceae). Chem Pharm Bull 38: 1624-1626.
- Artan M, Li Y, Karadeniz F, Lee SH, Kim MM, et al. (2008) Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava. Bioorg Med Chem 16: 7921-7926.
- Jiménez-Escrig A, Gómez-Ordóñez E, Rupérez P (2011) Seaweed as a Source of Novel Nutraceuticals: Sulfated Polysaccharides and Peptides. In SK. Kim (Ed.). Advances in Food and Nutrition Research Academic Press 64: 325-337.
- Kim SK, Wijesekara I (2017) Role of Marine Nutraceuticals in Cardiovascular Health. In D. Bagchi (Edn). Sustained Energy for Enhanced Human Functions and Activity. Academic Press 273-279 .
- Liu Z, Nakamura T, Munemasa S, Murata Y, Nakamura Y (2016) Galloylated Catechins as Potent Inhibitors of Angiotensin Converting Enzyme. Food Sci Technol Res 22: 847-851.
- Manandhar B, Paudel P, Seong SH, Jung HA, Choi JS (2019) Characterizing Eckol as a Therapeutic Aid: A Systematic Review. Mar Drugs 17: 361.
- Wright AE, Rueth SA, Cross SS (1991) An antiviral sesquiterpene hydroquinone from the marine sponge Strongylophora hartmani. J Nat Prod 54: 1108-1111.
- (NCIRD) (2020) Clinical Guidance Management Patients. National Center for Immunization and Respiratory Diseases, Diseases, Division of Viral.
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, et al. (2000) The Protein Data Bank. Nucleic Acids Res 28: 235-242.
- Berman HM, Henrick K, Nakamura H (2003) Announcing the worldwide Protein Data Bank. Nat Struct Biol 10: 980.
- Meng T, Cao H, Zhang H, Kang Z, Xu D, et al. (2020) The insert sequence in SARS-CoV-2 enhances spike protein cleavage by TMPRSS. bioRxix.
- Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, et al. (1983) A program for macromolecular energy, minimization, and dynamics calculations. J Compt Chem 4: 187-217.
- Ravindranath PA, Forli S, Goodsell DS, Olson AJ, Sanner MF (2015) Advances in protein-ligand docking with explicitly specified binding site flexibility. PLoS Comput Biol 11: e1004586.
- Al-Khafaji K, Tok T (2020) Understanding the mechanism of Amygdalin's multifunctional anti-cancer action using computational approach. J Biomol Struct Dyn 1-14.
- Auto GridFR (AGFR), a free graphical user interface for specifying the docking box. Retrieved from http://adfr.scripps.edu/AutoDockFR/agfr.html
- Al-Khafaji K, Tok T (2020) Amygdalin as multi-target anticancer drug against targets of cell division cycle: double docking and molecular dynamics simulation. J Biomol Struct Dyn 2: 1-10.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3-26.
- Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, et al. (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45: 2615-2623.
- Biovia DS (2016) Dassault Systemes BIOVIA, Discovery Studio. San Diego: Dassault Systèmes. Retrieved from https://www.3dsbiovia.com/about/citations-references.
- Egan WJ, Lauri G (2020) Prediction of intestinal permeability. Adv Drug Deliv Rev 54: 273-289.
- Oliveira LD, Davi M, Oliveira TD, Mota K (2020) Comparative Computational Study of SARS-CoV-2 Receptors Antagonists from Already Approved Drugs. Chemrxiv 10.
- Karadeniz FK, Park JW, Park SJ, Kim SK, Egan WJ (2014) Anti-HIV1 activity of phlorotannin derivative 8,4-dieckol from Korean brown alga Ecklonia cava. Biosci Biotechnol Biochem 78: 1151-1158.
- Egan WJ, Merz KM, Baldwin JJ (2000) Prediction of drug absorption using multivariate statistics. J Med Chem 43: 3867-3877.
- Jha A, Vimal A, Bakht A, Kumar A (2019) Inhibitors of CPH1-MAP Kinase Pathway: Ascertaining Potential Ligands as Multi-Target Drug Candidate in Candida albicans. Int J Pept Res Ther 25: 997-1010.
- Dong L, Hu S, Gao J (2020) Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 14: 58-60.
- Shi Q, Wang A, Lu Z, Qin C, Hu J, et al. (2017) Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr Res 453-454: 1–9.
- COVID-19 Molecular Structure and Therapeutics Hub. Retrieved from https://COVİD.bioexcel.eu/. 2020.
- Kong R, Wang F, Zhang J, Wang F, Chang S, et al. (2019) A Multistage Approach for Global and Site-Specific Protein-Protein Docking. J Chem Inf Model. 59: 3556-3564.
- Kong R, Yang G, Xue R, Liu M, Wang F, et al. (2020) COVID-19 Docking Server: An interactive server for docking small molecules, peptides and antibodies against potential targets of COVID-19. Bioinformatics.
- Trott O, Olson AJ, Vina AD (2010) Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31: 455-461.
- Weis WI, Drickamer K (1996) Structural Basis of Lectin-Carbohydrate Recognition. Annual Rev 65: 441-473.
- Lung J, Lin YS, Yang YH, Chou YL, Shu LH, et al. (2020) The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. Journal of medical virology 92: 693-697.
- Artan M, Li Y, Karadeniz F, Lee SH, Kim MM, et al. (2008) Anti-HIV-1 activity of phloroglucinol derivative, 6,6'-bieckol, from Ecklonia cava. Bioorg Med Chem 16: 7921-7926.
- Kang KA, Lee KH, Park JW, Lee NH, Na HK, et al. (2007) Triphlorethol-A induces heme oxygenase-1 via activation of ERK and NF-E2 related factor 2 transcription factor. FEBS Lett 581: 2000-2008.
- Kwon HJ, Ryu YB, Kim YM, Song N, Kim CY, et al. (2013) In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorg Med Chem 21: 4706-4713.