Leon DeJournett* and Jeremy DeJournett
Ideal Medical Technologies, USA
*Corresponding Author:
Leon DeJournett
MD, Founder, Ideal Medical Technologies USA.
Tel: 18283379960
E-mail: leondej@idealmedtech.com
Received date: April 25, 2017; Accepted date: May 03, 2017; Published date: May 10, 2017
Citation: DeJournett L, DeJournett J. Is Closed Loop Glucose Control for ICU Patients Just Around the Corner? J Intensive & Crit Care 2017, 3:2.
Effective glucose control in the Intensive Care Unit (ICU) setting has the potential to lower mortality rates [1], shorten length of stay [2] and decrease overall cost of care [3]. Yet the goal of achieving this control remains elusive due to the limitations of our current open loop methods [4] that still require manual testing of glucose values, entry of the measured value into local or web based glucose control software, and manual adjustment of the intravenous pumps infusing insulin into the ICU patient. In order to improve overall glucose control, ICU care givers will need to be empowered with a closed loop glucose control system.
The three main components of a closed loop glucose control system are a glucose sensor(s), control algorithm, and intravenous pump(s). Current intravenous pumps are accurate and reliable enough for a closed loop system, so the two components preventing completion of the system are the glucose sensor(s) and controller. An accurate and reliable glucose sensor array is a must, as trying to control a system without real time knowledge of mission critical sensor data will invariably lead to unacceptable outcomes, as has been seen in the aerospace industry [5]. In fact, the aerospace industry provides an excellent example of how to properly engineer a safe and effective control system, as they routinely build in redundancy of mission critical system components to improve reliability and will also use different methods of measurement to improve overall accuracy.
Studies on type I diabetics have shown that a multi-sensor array improves overall accuracy of the system [6]. The following equation can be used as a guide to determine the number of sensors N, needed given a known sensor failure rate p and a desired uptime rate q, where p, q ∈ [0,1]:
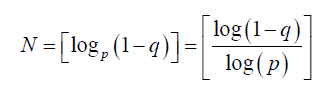
As can be seen from Table 1 if the closed loop system requirement is to have a complete sensor failure rate limited to less than 10 min over a typical 96 h ICU length of stay, which is a greater than 99.8% uptime rate, and the known sensor failure rate is 1%, then the system requirement is to have two independent glucose sensors in place.
Current CE marked blood based glucose sensors designed for use in the ICU setting have already been shown to be both highly accurate and reliable, with an uptime rate that exceeds 99% [7]. In addition, the next generation interstitial continuous glucose monitoring (CGM) system that Dexcom is developing with assistance from Google will not be affected by acetaminophen, making it a potential excellent candidate as the second sensor in a glucose array [8]. The ability to avoid interference from acetaminophen is important as this medication is known to affect the accuracy of current interstitial CGM systems, yet is ubiquitously used in the hospital setting. As sensor reliability is far more important than accuracy when it comes to closed loop glucose control [9], the improved uptime rate of a two sensor array will more than offset the decreased accuracy that will occur by averaging simultaneous blood and interstitial glucose values.
The second component of a closed loop system that is currently lacking is the control system. To date, the two main methods used to develop glucose control systems have been proportional integral derivative (PID) [10] and model predictive control (MPC) [11]. However, we have recently shown in a large scale simulation study that an artificial intelligence (AI) based controller may be capable of achieving results that are superior to those of both PID and MPC controllers [12]. These original results were recently corroborated in a comparative simulation study whereby the AI based controller was found to achieve overall results that were 76% better than PID [13]. The controller for a closed loop system should be able to iteratively cycle itself every 5-10 min in order to keep up with the highly nonlinear glucose-insulin system and should have proper safety features built in such as automatic recognition of spurious glucose sensor readings and a safe mode when all sensor readings are lost.
Although all of the components for a safe and effective closed loop glucose control system for ICU patients currently exist, the main impediment to bringing such a system to the market is lack of collaboration between the companies that have either already developed the necessary components [7,14] or are in an earlier stage in development [15]. If the best in class of available ICU based glucose sensors is combined with the best in class of available ICU based glucose controllers and the overall system is properly designed and engineered by a known engineering firm with class III medical device experience, the road to obtaining regulatory approval and bringing a safe and effective closed loop glucose control device to market will become much shorter.
| Sensor Failure rate |
Sensor # needed for uptime to exceed 95% |
Sensor # needed for uptime to exceed 99% |
Sensor # needed for uptime to exceed 99.9% |
Sensor # needed for uptime to exceed 99.999% |
| 0.1% |
1 |
1 |
2 |
2 |
| 1 |
1 |
2 |
2 |
3 |
| 2 |
1 |
2 |
2 |
3 |
| 5 |
2 |
2 |
3 |
4 |
| 10 |
2 |
3 |
4 |
6 |
Table 1 :Determines number of sensors needed to achieve a desired uptime rate given a known sensor failure rate.
References
- Krinsley JS, Preiser JC (2015) Time in blood glucose range 70 to 140 mg/dl >80% is strongly associated with increased survival in non-diabetic critically ill adults. Crit Care 19: 179.
- Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Tokumaru T, et al. (2014) Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care 37: 1516-1524.
- Cardona S, Pasquel FJ, Fayfman M, Peng L, Jacobs S, et al. (2017) Hospitalization costs and clinical outcomes in CABG patients treated with intensive insulin therapy. J Diabetes Complications 17: 742-747.
- Yamashita S, Ng E, Brommecker F, Silverberg J, Adhikari NK (2011) Implementation of the glucommander method of adjusting insulin infusions in critically ill patients. Can J Hosp Pharm 64: 333-339.
- Final Report (2012) Flight AF 447 Rio de Janeiro-Paris.
- Castle JR, Pitts A, Hanavan K, Muhly R, El Youssef J, et al. (2012) The accuracy benefit of multiple amperometric glucose sensors in people with type 1 diabetes. Diabetes Care 35: 706-710.
- Leopold JH, van Hooijdonk RT, Boshuizen M, Winters T, Bos LD, et al. (2016) Point and trend accuracy of a continuous intravenous microdialysis-based glucose-monitoring device in critically ill patients: A prospective study. Ann Intensive Care 6: 68.
- Dexcom (2016) Personal Communication.
- Wilinska ME, Hovorka R (2014) Glucose control in the intensive care unit by use of continuous glucose monitoring: What level of measurement error is acceptable? Clin Chem 60: 1500-1509.
- Steil GM (2013) Algorithms for a closed-loop artificial pancreas: The case for proportional-integral-derivative control. J Diabetes Sci Technol 7: 1621-1631.
- Bequette BW (2013) Algorithms for a closed-loop artificial pancreas: The case for model predictive control. J Diabetes Sci Technol 7: 1632-1643.
- DeJournett L, DeJournett J (2016) In silico testing of an artificial-intelligence-based artificial pancreas designed for use in the intensive care unit setting. J Diabetes Sci Technol 10: 1360-1371.
- DeJournett J, DeJournett L (2017) Comparative simulation study of glucose control methods designed for use in the intensive care unit setting via a novel controller scoring metric. J Diabet Sci Technol.
- Crane BC, Barwell NP, Gopal P, Gopichand M, Higgs T, et al. (2015) The development of a continuous intravascular glucose monitoring sensor. J Diabetes Sci Technol 9: 751-761.
- DeJournett L (2016) Artificial intelligence based artificial pancreas system in an animal model of stress induced hyperglycemia. Diabetes 65: A259.