- (2012) Volume 13, Issue 2
Muhammad Wasif Saif1, John Ng1, Bryan Chang2, Suzanne Russo3*
1Columbia University College of Physicians and Surgeons. New York, NY, USA
2Yale School of Medicine. New Haven, CT, USA.
3Mitchell Cancer Institute, University of South Alabama. Mobile, AL, USA
Pancreatic neuroendocrine tumors (PNET) represent a heterogeneous group of tumors with varying tumor biology and prognosis. Advanced PNETs remain a difficult therapeutic challenge because of their high malignant potential and their resistance to conventional chemotherapy although there have been recent developments with promising results with the use of novel agents for the treatment of this disease. Combined modality chemoradiation is not widely used in the management of locally advanced pancreatic endocrine tumors. We discuss Abstract #335 from 2012 ASCO GI Cancers Symposium and share our experience to discuss efficacy and toxicity of concurrent capecitabine or infusional 5-fluorouracil and radiotherapy in patients with resected, locally advanced and metastatic PNET. Prospective studies to investigate the role of radiation and chemoradiation are warranted.
Chemoradiotherapy; Neuroendocrine Tumors; Pancreas
Pancreatic neuroendocrine tumors (PNETs) represent a heterogeneous group of tumors with varying tumor biology and prognosis [1]. The incidence of PNETs has increased over the past two decades to approximately 5/1,000,000 persons. Advanced PNETs remain a difficult therapeutic challenge because of high malignant potential and resistance to conventional chemotherapy [2]. As a result, there are limited effective treatment options for patients with advanced disease.
There have been recent new developments with promising results with the use of novel molecular targeted agents for the treatment of this disease [3, 4, 5]. Traditional conventional chemotherapy agents included regimens based on etoposide, platinum agents, anthracyclines, streptozocin, and 5-fluorouracil (5-FU) based agents [6].
Combined modality chemoradiation is not widely used in the management of locally advanced pancreatic endocrine tumors.
Our colleagues at the University of Maryland School of Medicine, Baltimore, MD, USA and Johns Hopkins University School of Medicine, Baltimore, MD, USA presented a series of 11 patients with histologically confirmed PNET (T3/T4) who received external beam radiation therapy to the primary tumor or resection bed to a median dose of 50.4 Gy (Abstract #335 [7]). Of these 11 patients, 7 received concurrent capecitabine (1,000 mg/m2 bid). Among 9 patients with locally advanced disease, two were able to undergo surgical resection. At a median follow-up of 30.4 months, 3 patients were dead with progressive disease, 2 had died without progressive disease, 3 were alive with metastases, and 3 were alive without metastases (1 stable, 1 partial response, 1 complete response). Only two grade 3 toxicities were noted. The authors concluded that local radiation therapy may convert initially unresectable locally-advanced tumors to disease amenable to surgical resection, which would theoretically improve local control [7].
To further augment these data, we present our experience in treating patients with PNET with chemoradiation.
We reviewed the medical records of patients with PNET who received treatment with either capecitabine or 5-FU and irradiation. Table 1 summarizes the patients’ characteristics. Six patients (2 females and 4 males) with PNET treated with capecitabine or 5-FU and radiation were identified. Median age of the patient population was 52 years (range: 38 to 63 years). All 6 patients had biopsy proven PNET. One patient had tumor resected with negative margins, 2 patients underwent surgery with positive margins, and 3 patients had unresectable locally advanced disease. Most patients were of Eastern Cooperative Oncol ogy Group (ECOG) performance status 0 to 1, with grade 0 to 1 weight loss, and grade 0 to 1 pain associated with disease. No patients had prior treatment for malignancy. Four patients were treated with capecitabine (median dose 600 mg/m2 po bid; range: 600-800 mg/m2) and 2 patients were treated with infusional 5-FU (175 mg/m2/day) with concurrent radiation therapy. Radiotherapy began on the first day of week 1 of capecitabine or 5-FU. The target volume received external beam radiation at 180 Gy/day delivered Monday through Friday for a total dose of 50.4 Gy. The treatment volume consisted of the gross tumor volume (GTV), defined by pancreatic and locoregional radiographic abnormalities identified by contrast-enhanced computed tomography (CT), the clinical target volume (CTV) defined as the area at risk for subclinical microscopic disease, and the planning target volume (PTV), typically consisting of a 0.5 cm margin outside of the clinical target volume.
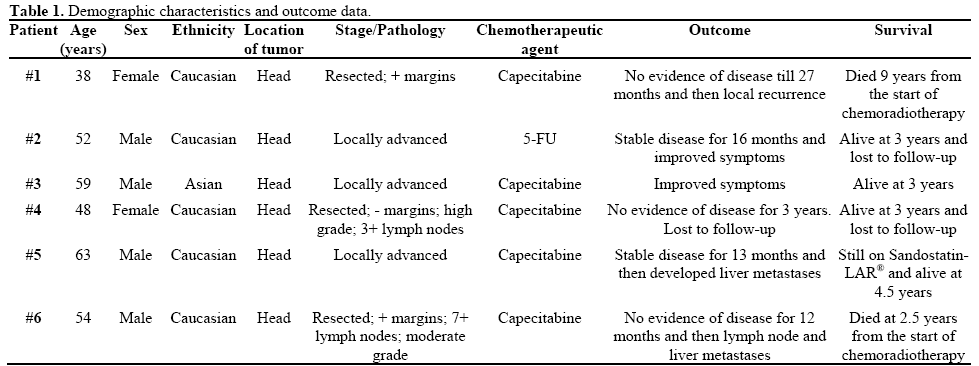
All patients completed the full course of chemoradiotherapy at the intended dose without a break or dose reduction of therapy, with the exception of one patient in whom 5-FU was held for 3 days due to grade 3 diarrhea. Two patients developed grade 2 hand-foot syndrome: one during week 4 who required dose reduction from 800 mg/m2 to 600 mg/m2 and the second patient developed after finishing the chemoradiation. The patient who received 5-FU developed grade 1 mucositis and no patient developed grade 3, or more, hematological toxicity.
In our series, all 6 patients experienced clinical benefit and partial radiographic response. One patient survived 9 years from following treatment and another patient is alive at 4.5 years but good disease control only on Sandostatin-LAR®. Furthermore, the combined modality treatments were well tolerated with minimal toxicity.
Our results are in agreement with the report presented at the 2012 ASCO GI Cancers Symposium [7]. Review of literature showed a previous report by Strosberg et al. [8] in which 6 patients were treated with induction chemotherapy followed by concurrent radiation with infusional 5-FU or capecitabine. An objective radiographic response rate was observed in 80% of patents and the chemoradiation was well-tolerated.
It appears that the synergistic effect of radiosensitization of 5-FU based agents and concomitant radiotherapy provides excellent local control in the setting of locally advanced PNETs. As we make strides toward discovering the biology behind PNETs we may be able to better understand the apparent radiosensitivity of these tumors [9]. Furthermore, as radiotherapy techniques evolve to enable high doses to be delivered in this anatomic region, dose escalation may further improve the therapeutic window of chemoradiation [10].
The recent success of the biologic targeted agents, such as everolimus and sunitinib, has renewed excitement about treating advanced PNETs. Testing these agents with radiation therapy may also provide another option in a disease that historically has had few treatment options.
The authors have no potential conflict of interest