- (2012) Volume 13, Issue 5
Parvin F Peddi1, Sam Lubner5, Robert McWilliams6, Benjamin R Tan1, Joel Picus1, Steven M Sorscher1, Rama Suresh1, A Craig Lockhart1, Jian Wang7, Christine Menias2, Feng Gao3, David Linehan4, Andrea Wang-Gillam1
1Division of Medical Oncology, Department of Medicine, 2Division of Diagnostic Radiology, Mallinckrodt Institute of Radiology, 3Division of Biostatistics, and 4Section of Hepatobiliary and Pancreatic Surgery, Division of General Surgery; Washington University School of Medicine. Saint Louis, MO, USA. 5Division of Medical Oncology, Department of Medicine, University of Wisconsin School of Medicine and Public Health. Madison, WI, USA. 6Division of Medical Oncology, Mayo Clinic. Rochester, MN, USA. 7Division of Oncology, The Second Affiliated Hospital of Zhengzhou University. Zhengzhou, Henan, China
Received June 7th, 2012 - Accepted July 10th, 2012
Context Combination chemotherapy with FOLFIRINOX (oxaliplatin, irinotecan, fluorouracil, and leucovorin) was shown to beeffective in a large phase III trial. Objective The purpose of this study was to examine the tolerance and effectiveness ofFOLFIRINOX as practiced outside of the confines of a clinical trial and to document any dose modifications used by practicing oncologists. Methods Data on patients with all stages of pancreatic adenocarcinoma treated with FOLFIRINOX at three institutions was analyzed for efficacy, tolerance, and use of any dose modifications. Results Total of 61 patients was included in this review. Median age was 58 years (range: 37 to 72 years), 33 were male (54.1%) and majority had ECOG performance of 0 or 1 (86.9%, 53 patients). Thirty-eight (62.3%) had metastatic disease, while 23 (37.7%) were treated for locally advanced or borderline resectable disease. Patients were treated with a median number of four cycles of FOLFIRINOX, with dose modifications in 58.3% (176/302) of all cycles. Ten patients had stable disease (16.4%), four had a partial response (6.6%) while eight had progressive disease (13.1%) on best imaging following therapy. Median progression-free survival and overall survival were 7.5 months and 13.5 months, respectively. The most common grade 3-4 adverse event was neutropenia at 19.7% (12 cases), with 4.9% (3 cases) rate of febrile neutropenia. Twenty-one patients (34.4%) were hospitalized as a result of therapy but there were no therapy-related deaths. Twentythree (37.7%) had therapy eventually discontinued as a result of adverse events. Conclusion Despite substantial rates of adverse events and use of dose modifications, FOLFIRINOX was found to be clinically effective in both metastatic and non-metastatic patients. Regimen toxicity did not detract from overall response and survival.
Adenocarcinoma; Adult; Antimetabolites, Antineoplastic /adverse effects; Antineoplastic Combined Chemotherapy Protocols /adverse effects; Follow-Up Studies; Granulocyte Colony-Stimulating Factor; Neoadjuvant Therapy; Pancreatic Neoplasms; Registries; Survival Analysis; United States
ACCORD 11/PRODIGE 4: Action to Control Cardiovascular Risk in Diabetes 11/Partenarait de Recherche en Oncologie Digestive 4; ECOG: Eastern Cooperative Oncology Group; FOLFIRINOX: oxaliplatin, irinotecan, fluorouracil, and leucovorin; FOLFOX: oxaliplatin, fluorouracil, and leucovorin; GCSF: granulocyte colony stimulating factor
Pancreatic cancer remains the fourth leading cause of cancer death in the U.S. with an estimated 37,390 deaths in 2012 [1]. Progress in combating this malignancy has been slow with five year overall survival improving from 3% in 1970s to merely 6% in the early 2000s [1]. Until recently, the only chemotherapy shown to provide a modest survival benefit had been gemcitabine, which improved median overall survival from 4.4 to 5.6 months when compared to fluorouracil in a landmark phase III study [2]. Attempts at improving survival with combination of gemcitabine and a variety of cytotoxic and molecularly targeted agents have failed to provide substantial additional benefit [3, 4, 5]. Addition of erlotinib to gemcitabine was the only combination to provide a modest additional survival benefit of 6% at 1 year, resulting in an FDA approval for this agent [6].
In May 2011, Conroy et al. published the results of the Action to Control Cardiovascular Risk in Diabetes 11 / Partenarait de Recherche en Oncologie Digestive 4 (ACCORD 11/PRODIGE 4) trial on FOLFIRINOX, as the first regimen to improve the median overall survival of patients with metastatic pancreatic cancer beyond 10 months [7]. FOLFIRINOX, consisting of a biweekly regimen of oxaliplatin, irinotecan, fluorouracil, and leucovorin, was compared in a phase II to III converted trial with gemcitabine in 342 patients in various European centers. Median overall survival was 11.1 months compared with 6.8 months in the gemcitabine group. Furthermore, in spite of higher rates of adverse events, patients in the FOLFIRINOX group reported a higher quality of life.
In spite of the improvement in survival, however, acceptance of FOLFIRINOX has been tempered with toxicity concerns of this potent regimen. A similar regimen (FOLFOXIRI: irinotecan 165 mg/m2 day 1 instead of 180 mg/m2 in FOLFIRINOX, same dose of oxaliplatin at 85 mg/m2 day 1, leucovorin at 200 mg/m2 day 1, and fluorouracil at 3,200 mg/m2 48-hour continuous infusion instead of 2,400 mg/m2 in FOLFIRINOX) was tried in colon cancer and has not been widely accepted partly due to similar toxicity concerns [8]. We therefore sought to document the use of FOLFIRINOX and its efficacy and tolerance in patients treated at three U.S. academic institutions within the framework of a registry study.
Patient Population
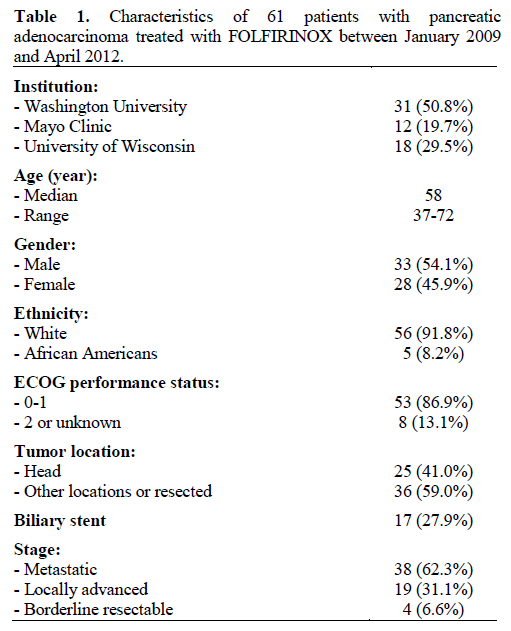
Patients with pancreatic adenocarcinoma at Washington University in St. Louis Siteman Cancer Center, Mayo Clinic, and University of Wisconsin Carbone Cancer Center, who were treated with FOLFIRINOX between January 2009 and April 2012, were eligible for inclusion. A retrospective and prospective registry was established to capture all patients after Institutional Board Review (IRB) approval was obtained at each institution. Patients were 18 years of age or older and had pathologically confirmed pancreatic adenocarcinoma. Treatment with at least one cycle of a regimen consisting of 5- fluorouracil, oxaliplatin, and irinotecan was required for entry into the registry. Patients with all stages of pancreatic cancer and prior treatment histories were eligible. Demographic data collected included age, race, gender, Eastern Cooperative Oncology Group (ECOG) performance status, stage at initiation of FOLFIRINOX, and any prior treatments. Baseline laboratory values were recorded including tumor marker CA 19-9. Data collection was stopped in March 2012.
Drug Administration
The standard FOLFIRINOX regimen has been previously described in the ACCORD 11 trial [7], consisting of oxaliplatin at 85 mg/m2, leucovorin at 400 mg/m2, irinotecan at 180 mg/m2, and fluorouracil at 400 mg/m2, followed by a continuous fluorouracil intravenous infusion of 2,400 mg/m2 over a 46-hour period every 2 weeks. Any deviation from this regimen, whether in the first or subsequent cycles, was recorded. The use of granulocyte colony stimulating factor (G-CSF) support, given to prevent or decrease levels of neutropenia, was recorded for each cycle. Patients were followed until cessation of FOLFIRINOX per the treating oncologist. The total number of cycles received was recorded.
Evaluation of Clinical Toxicity
All clinic notes were reviewed for any hospitalizations, dose reductions or cessation of treatment due to adverse events. Laboratory values before and after each treatment cycle were also reviewed to corroborate and document any additional adverse events using complete blood count and comprehensive metabolic panel in each patient. Adverse events were graded using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, version 3.0) [9]. Most common adverse events specifically tabulated in the paper include neutropenia, neutropenic fever, thrombocytopenia, anemia, fatigue, nausea, abdominal pain, and diarrhea. Patients who received at least one cycle of chemotherapy were included for toxicity evaluation.
Assessment of Clinical Benefit
Staging imaging (CT or MRI) was obtained per the discretion of treating oncologists during treatment. These images were analyzed according to the Response Evaluation Criteria in Solid Tumors (RECIST) as described in the supplement of the ACCORD trial to determine response [7]. Images were included in response analysis if patients had received at least three cycles of a standard or modified FOLFIRINOX regimen prior to image acquisition. Original report by radiology was used for patients treated at Mayo Clinic and University of Wisconsin. An independent radiologist reviewed images of patients at Washington University, for the purposes of this study. Best response imaging was used for the purpose of determining clinical benefit. Clinic notes were also reviewed for clinician assessment of response and when it differed from radiological assessment, the clinical assessment of response was used.
Institutional Board Review (IRB) approval was obtained at each institution. Written or oral informed consent was obtained from each patient. The study protocol conforms to the ethical guidelines of the "World Medical Association (WMA) Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects" adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended by the 59th WMA General Assembly, Seoul, South Korea, October 2008.
Data analysis for this study was descriptive in nature. Demographic and clinical characteristics, as well as adverse events and follow-up time were summarized using means, medians, and standard deviations, ranges or counts and frequencies as appropriate. Progressionfree survival was defined as time of FOLFIRINOX initiation to time of progression based on imaging/clinical evaluation, or time of death, whichever occurred first. Those patients alive and progression-free were censored on March 1st, 2012, the date of data collection close. Overall survival was defined as time of FOLFIRINOX initiation to time of death. When not noted in clinical records, the Social Security Death index was used to ascertain survival and patients alive were censored in March 2012. The overall and progression-free survival were calculated using Kaplan-Meier product limit methods. Statistical analyses were performed using SAS (SAS Institutes, Cary, NC, USA).
Patients
Patient characteristics are shown in Table 1. A total of 61 patients were enrolled and followed for a median of 8.5 months (range of 1.5 to 20.4 months). Median age was 58 with a range of 37 to 72 years. Majority of the patients were male (54.1%), white (91.8%) and with ECOG performance of 0 or 1 (86.9%). Thirty-eight (62.3%) patients were treated for metastatic or recurrent disease, while 19 (31.1%) had locally advanced cancer and 4 had border-line resectable disease (6.6%). Twenty-five patients (41.0%) had tumor present in the pancreatic head at time of receiving FOLFIRINOX with 17 patients (27.9%) having a biliary stent.
Treatment
Patients received a median of 4 cycles of FOLFIRINOX with range of 1 to 22 cycles. Average number of cycles was 5.4. Three patients started with one cycle of FOLFOX (same dosage and schedule of oxaliplatin, 5-FU, and leucovorin as FOLFIRINOX, but omitting irinotecan) to test tolerability before the addition of irinotecan. This first cycle of FOLFOX was not counted towards cycle count or analysis.
The FOLFIRINOX regimen was modified in 31 patients (50.8%) empirically starting with the first cycle. Deletion of 5-FU and dose reduction of irinotecan were the most common modifications and were each selected for 22 patients with some overlap of the two groups. Thirty-two patients had UTG1A1 status evaluated and dose reduction of irinotecan was done in 4 patients due to presence of UGT1A1*28/28 genotype. Median dose intensities for the first cycle for each of the components were 100%. Average dose intensities for the first cycle for oxaliplatin, irinotecan, 5-FU bolus, and 5-FU continuous infusion were 98%, 90%, 59%, 99%, respectively. Overall dose averages for oxaliplatin, irinotecan, 5-FU bolus, and 5-FU continuous infusion were 96%, 88%, 57%, and 96%, respectively. From the 31 patients who started with full dose FOLFIRINOX, 20 (64.5%) required eventual dose reduction or discontinuation of therapy due to adverse events.
G-CSF support was included for 41 patients (67.2%) starting with the first cycle using filgastrim. Except for three patients in whom G-CSF support was later discontinued, all others were continued on G-CSF for all subsequent cycles. On the other hand, from the twenty patients who were not started on G-CSF with first cycle, 6 (30.0%) had G-CSF support added later on. Overall 47 patients (77.0%) received G-CSF support at some point in their therapy.
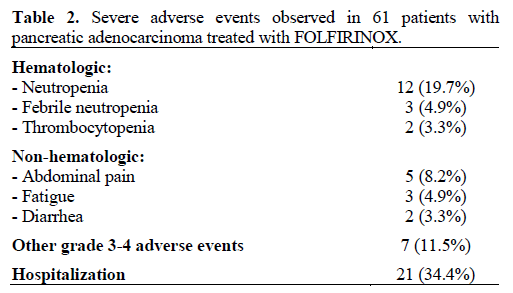
Adverse Events
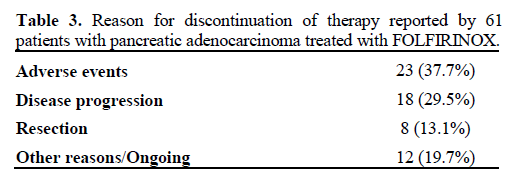
There were no deaths as a result of therapy. In six patients (9.8%) treatment was stopped after the first cycle due to adverse events. Rates of the most common grade 3-4 adverse events are listed in Table 2. Neutropenia was the most common grade 3-4 adverse event, occurring in 12 patients (19.7%), while neutropenic fever occurred in three patients (4.9%). Two of these patients were already on G-CSF prophylaxis but were on full dose FOLFIRINOX therapy. Grade 3 or worse thrombocytopenia occurred in two patients (3.3%) while no patient had a grade 3-4 anemia. Abdominal pain, fatigue and diarrhea were the severe non-hematological adverse events that were observed. Intravenous fluids and anti-diarrhea medications as outpatient usually sufficed for treatment but hospitalizations were required for a total of 21 patients (34.4%). There was one case of cholangitis whose tumor was in the pancreatic head and did not have a stent. No definite correlation was observed between adverse events and UGT1A1 genotype. Adverse events caused dose reduction in 23 patients (37.7%) and resulted in the eventual reason for discontinuation of FOLFIRINOX in 23 patients (37.7%). Overall eleven patients (18.0%) received full dose FOFIRINOX throughout their treatment without adverse events necessitating dose reduction or cessation. Reason for eventual discontinuation of FOLFIRINOX therapy is depicted in Table 3.
Clinical Outcome
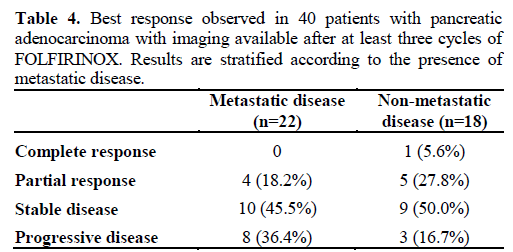
Forty patients (65.6%) had imaging available after at least three cycles of FOLFIRINOX therapy: 22 with metastatic disease (57.9%) and 18 with non-metastatic disease (78.3%). Overall, one patient had complete response (2.5%), nine patients had a partial response (22.5%), 19 had stable disease (47.5%), and 11 had disease progression (27.5%) (Table 4). All four patients with borderline resectable disease receiving FOLFIRINOX were able to undergo resection afterwards. From the 19 patients with locally advanced disease, 4 (21.1%) were also able to undergo resection after receiving radiation following FOLFIRINOX therapy. From the 18 patients with non-metastatic disease who had imaging available, 9 (50.0%) had stable disease, 5 (27.8%) partial response and 1 (5.6%) had a complete response post treatment with FOLFIRINOX. From the 22 patients with metastatic disease, 14 (63.5%) had either partial response or stable disease.
A total of 23 patients died (37.7%) and 29 patients progressed (47.5%). Median progression-free survival was 7.5 months, with 1-year progression-free survival of 35.3%. Overall survival was recorded using medical records and Social Security Death index for all patients. Median overall survival was 13.5 months, with 1-year overall survival of 55.6% (Figure 1). Oneyear overall survival for patients with metastatic disease was 41.8% compared with 75.5% without metastasis.
This is the first study to our knowledge examining FOLFIRINOX use outside of a clinical trial after the ACCORD study in U.S. patients with pancreatic adenocarcinoma [10]. FOLFIRINOX was found to be widely adopted at the centers examined in this study, not only in patients with metastatic disease who were the patient population studied in the ACCORD trial, but also in locally advanced and borderline-resectable disease.
Given concerns for toxicities, FOLFIRINOX dosage was frequently adjusted empirically as well as in response to adverse events. In fact half of the patients were given reduced dosing of FOLFIRINOX starting with the first cycle before the development of any adverse events. Nonetheless, a significant portion of the patients (34.4%) were hospitalized due to adverse events. The rate of hospitalizations was not reported in the ACCORD trial. Only one case of cholangitis was seen, which is reassuring given the high propensity of stents in this patient population. A higher proportion of patients received G-CSF compared to the ACCORD trial (77.0% vs. 42.5%). Accordingly, rates of grade 3-4 neutropenia were lower in our patient population (19.7% vs. 45.7%) but rates of neutropenic fever were similar in both studies at less than 5%.
In spite of dose reductions, FOLFIRINOX was found to be very effective. Rates of response were similar to the ACCORD study with more than 70% of patients having at least stable disease on therapy. Similar response rates were seen in non-metastatic patients who were not included in the ACCORD trial. In fact, 8 patients were able to undergo resection after FOLFIRINOX therapy. This is notable given the extensive desmoplastic stroma reaction in localized disease and concern for reduced efficacy of chemotherapy in this setting. Progression-free and overall survivals were longer than the results published in the ACCORD trial (7.5 months and 13.5 months, respectively) likely reflecting inclusion of patients with non-metastatic disease. One-year survival rate for patients with metastatic disease was 41.8%, similar to the 48.4% reported in the ACCORD trial.
The strength of this study is that it provides real world data on the use of FOLFIRINOX in patients with pancreatic cancer. Moreover, it provides the first data on patients treated in the U.S.. In addition, it includes patients with non-metastatic disease that were not included in the original ACCORD trial. The use of FOLFIRINOX in these patients has been reported in at least one other retrospective study and is promising [11]. One weakness of this study is that dose reductions were not protocol driven and instead were up to the discretion of the treating oncologists. As a result, it is difficult to judge if all treatment modifications were performed in a standard fashion or were clinically necessary. Similarly, not all physicians obtained tumor markers at regular intervals and therefore our study did not attempt to correlate tumor marker levels with response. Another weakness of this study is that the median age of patients at 58, while similar to the ACCORD study, was much younger than median age of 71 observed in the SEER database [12]. It is therefore important to note that it is not known whether older patients would tolerate this regimen. In regards to the use of G-CSF, rates of febrile neutropenia seen in this study do not justify use of growth factors per NCCN guidelines. However, given that 77.0% of patients received growth factor support, it could be argued that the rates of febrile neutropenia were low only as a result. It may therefore be reasonable to include G-CSF support with this regimen and later discontinue it if not needed.
In conclusion, we believe it is acceptable to use FOLFIRINOX instead of gemcitabine monotherapy in future clinical trials as the new gold standard in patients with pancreatic cancer given its efficacy and acceptable rate of adverse events. It remains to be seen how FOLFIRINOX compares to other regimens currently in clinical trials, such as combination of gemcitabine with nab-paclitaxel, which has had promising results in phase I/II trials [13]. It is also not known whether FOLFIRINOX can serve as a backbone chemotherapy to which targeted agents, such as erlotinib, could be added similarly to what had been done with gemcitabine. Increased toxicity may preclude addition of other agents to FOLFIRINOX.
Use of UTG1A1 testing and appropriate irinotecan dose reduction may be useful in reducing at least some of the toxicities. At the present time, however, FOLFIRINOX by itself is more effective than any other regimen in patients with pancreatic cancer and its toxicities did not preclude its use in the patients studied in this review.
The author has no potential conflict of interest