Research Article - (2023) Volume 8, Issue 6
Multiplicative and Additive Interactions of Hyperuricemia and Hypertension on the Risk of Chronic Kidney Disease: Evidence from a Prospective Population-Based Cohort Study
Zhibin Ma1,
Xiaobin Hu2*,
Ping Hu1,
JunLing Shi1,
Yajjun Wang1,
Yingli Tian1,
Hong Liu1,
Feng Xu1 and
Mengli Nan1
1Xi ‘an No 1 Hospital, Xian, Shanxi Province 730000, China
2Department of Epidemiology and Health Statistics, Lanzhou University, China
*Correspondence:
Xiaobin Hu,
Department of Epidemiology and Health Statistics, Lanzhou University,
China,
Email:
Received: 04-Dec-2023, Manuscript No. IPJHCC-23-18503;
Editor assigned: 06-Dec-2023, Pre QC No. IPJHCC-23-18503 (PQ);
Reviewed: 20-Dec-2023, QC No. IPJHCC-23-18503;
Revised: 25-Dec-2023, Manuscript No. IPJHCC-23-18503 (R);
Published:
01-Dec-2024, DOI: 10.36846/2472-1654-8.6.8060
Abstract
Objective: To investigate the association between hyperuricemia (HUA) and the risk of Chronic Kidney Disease (CKD) in the Jinchang cohort and the interaction between HUA and hypertension on the risk of CKD, in order to provide a scientific basis for the prevention and treatment of CKD.
Methods: Based on the Jinchang cohort, a Cox regression model was applied to investigate the association between HUA and the risk of CKD using the non-HUA population as a reference, and HR values (95% CI) were calculated. According to the baseline age (<45 years, 45 years-64 years, ≥ 65 years), gender (male, female), BMI (<24.0 kg.m-2, 24.0 kg.m−2-27.9 kg.m-2, ≥ 28.0 kg.m-2), smoking (no, yes, quit smoking), alcohol consumption (no, yes, quit drinking, the study population diabetes (no, yes) hypertension (no, yes) and occupation (worker, other) were analyzed by subgroups. The product term of HUA and hypertension was also added to the Cox regression model to test whether there was a multiplicative interaction between the association of HUA and hypertension with the risk of developing CKD. The additive interaction between HUA and hypertension on the risk of developing CKD was examined using SAS macros, and the relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP), and The results showed that after adjusting for the confounding factors, the H-risk ratio was higher than the RERI.
Results: After adjusting for confounders, there was an association between HUA and the risk of developing CKD, with the risk of CKD in the HUA population being 1.28 times higher than in the non-HUA population (HR=1.28, 95% CI:1.12-1.47). This association was more pronounced in the age ≥ 65 years, female, BMI <24 kg.m-2, ex-smoker, worker, non-diabetic and hypertensive populations. The interaction results showed a positive multiplicative and additive interaction between HUA and hypertension on the risk of developing CKD. The product term INTM (95% CI) for HUA and hypertension was 1.33 (1.01-1.77); the additive interaction evaluation indexes RERI (95% CI), AP (95% CI), and SI (95% CI) were 0.64 (0.21-1.07), 0.27 (0.11-0.43), and 1.93 (1.16-3.19), respectively.
Conclusion: HUA is a risk factor for the development of CKD and has a synergistic effect with hypertension on the development of CKD. For the early prevention and treatment of CKD, the focus should be on and intervention for those with HUA combined with hypertension, so that the limited health resources and funds can be used rationally to the maximum extent.
Keywords
Hyperuricemia; Chronic kidney disease; Interaction; Cohort study
Introduction
Chronic Kidney Disease (CKD) is defined as a glomerular
filtration rate (GFR) <60 mL min−1 1.73 m−2 or the presence
of one or more markers of renal injury for at least 3 months,
which include albuminuria, abnormal urine sedimentation,
and histological or structural abnormalities of the kidney [1].
Research shows that the global prevalence of CKD and the
number of people with disabilities and deaths caused by CKD are
increasing year by year. In 2017, the global total prevalence of
CKD was 9.1%, and the number of CKD patients reached nearly
700 million, more than the number of patients with diabetes,
osteoarthritis, chronic obstructive pulmonary disease, asthma
or depression, and one third of the patients are in China and
India [2,3]. Research has shown that from 1990 to 2017, the
global all-age mortality rate of CKD increased by 41.5%. In 2017,
1.2 million people worldwide died from CKD, and the ranking
of causes of death has also increased from 17th in 1990 to 12th [2]. CKD is also one of the urgent public health issues to be
addressed in China. Research shows that the prevalence rate
of CKD in China was 10.8% in 2009, and 8.2% in 2018-2019 [4].
From the prevalence rate, although the prevalence rate of CKD
in China has declined over the past decade, the awareness rate
of CKD is still very low, only 10%. Among CKD patients receiving
treatment, the control rate of hypertension, diabetes and
dyslipidemia is very low, 24.5%, 42.3% and 31.5% respectively
[5]. Although the etiology of CKD is not yet clear, identifying risk
factors for CKD and intervening can help prevent its occurrence
or delay its progression, thereby reducing the risk of death and
reducing the burden of CKD disease [6]. Research has shown
that HUA can not only predict the risk of cardiovascular disease
and metabolic syndrome, but also be an independent risk
factor for CKD, and the risk of CKD increases with the increase
of sUA [7-9].
Importantly, the occurrence and progression of CKD are the
result of the long-term accumulation of multiple etiologies and
risk factors, which can act independently or jointly on the body,
producing synergistic or antagonistic effects [10]. For example,
a cohort study in Taiwan, China shows that the association
between HUA and sUA and CKD is stronger in women, and both
HUA and sUA have a positive multiplicative interaction with
gender on CKD [11]. A cohort study in the Netherlands showed
that the association between sUA and the decrease in CKD and
eGFR was more pronounced in hypertensive populations [12],
and there was a positive multiplicative interaction between
sUA and hypertension on the occurrence of CKD and the
decrease in eGFR. However, the above studies only explored
the multiplicative interaction between HUA or sUA and gender,
hypertension, and did not further analyze whether there is an
additive interaction. Moreover, research on the interaction
between HUA and other related factors on the onset of CKD is
still very limited. Therefore, this study is based on the Jinchang
queue platform and uses the SAS interaction macro to explore
the interaction between HUA and hypertension on the risk of
CKD, providing scientific basis for the prevention and treatment
of CKD [13].
Materials and Methods
Study Design and Participants
The participants were all drawn from the Jinchang cohort
[14-16], an ongoing prospective cohort study in Jinchang
City, Gansu Province, China, based on the biennial physical
examination of all employees of Jinchuan Nonferrous Metals
Company (JNMC). From June 2011 to December 2013, a total
of 48,001 participants completed the cohort baseline survey,
and 33,355 participants completed the first round of follow-up
survey from January 2014 to December 2015, with a median
follow-up time of 2.2 years.
From the 33355 study subjects who completed the first round
of follow-up, 179 cases of self-reported kidney disease, 1560
cases of missing CKD diagnostic information at baseline and
follow-up, 1128 cases of baseline CKD patients, 146 cases of
baseline malignant tumors, 179 cases of baseline gout patients,
and 1741 cases of missing sUA at baseline and follow-up were
excluded from the baseline epidemiological survey. Finally,
28422 study subjects were included.
Data Collection
The research data used in this study were derived from the
Jinchang cohort baseline survey and the first round of followup
surveys, including epidemiological questionnaires, physical
examinations, and clinical biochemical examinations. Our
research team designed the standardized and structured
epidemiological questionnaires to collect basic sociodemographic
information (age, gender, education, occupation,
etc.), behavioral characteristics (smoking, drinking, exercise,
etc.), and medical history of the participants. Uniformly trained
interviewers conducted the questionnaire survey through oneon-
one and face-to-face interviews. During the survey, it was
ensured that the respondents clearly understood the content
of the questionnaire, avoiding inducing questions, and crosschecking
was conducted after completing the survey.
The physical examination and clinical biochemical examination
were completed by the clinical staff of the Workers’ Hospital
of the Jinchuan Company, including height, weight, blood
pressure, and various clinical biochemical indexes. Height and
weight were measured by a computerized body scale (SK-X80/
TCS-160D-W/H, Sonka, China) when the participants took off
their shoes and wore light clothes. Body mass index (BMI) was
calculated as weight in kilograms divided by height in meters
squared (kg.m−2). The blood pressure in a sitting position
was measured by an electronic sphygmomanometer (BP750,
AMpall, Seoul, Korea) three times continuously after at least
10 minutes of rest, and the average values were taken. Before
venous blood collection, all participants were instructed to
fast for at least 8 h. The clinical biochemical examination
was detected by an automatic biochemical analyzer (Hitachi
7600-020, Kyoto, Japan), mainly including serum creatinine
(Scr), sUA, total cholesterol (TC), fasting plasma glucose (FPG),
triglyceride (TG), high-density lipoprotein cholesterol (HDL-C),
and low-density lipoprotein cholesterol (LDL-C).
Study Outcomes and Related Definitions
CKD was defined in this study as the presence of abnormal
glomerular filtration rate (eGFR <60 mL min−1 1.73 m−2) or
proteinuria (urine dipstick reading ≥ 1+), of which eGFR
was estimated according to the Chronic Kidney Disease
Epidemiology Collaboration equation (CKD-EPI), based on Scr,
age, and gender [1,17].
Covariates
Smokers were those who smoked at least one cigarette a day
for more than 6 months, and non-smokers were those who
never smoked or who smoked occasionally but did not meet
the definition of a smoker. Ex-smokers were those who used to
smoke but had not smoked for more than 6 months. Drinkers
were those who drank liquor or other spirits, wine or other fruit
wine, beer, and other alcohol at least once a week for more than
6 months, and non-drinkers were those who never drank or
drank occasionally but did not meet the definition of drinkers.
Ex-drinkers were those who used to drink but had not drunk
for more than 6 months. Physical exercise was divided into
three types: No, occasionally, and often exercise. Occasionally
exercise was defined as exercise less than 3 times a week and
exercise more than 30 minutes on average, and often exercise
was considered as exercise at least 3 times a week for more
than 30 minutes each time. Hypertension was defined as selfreported
physician-diagnosed hypertension or definite clinical
records of hypertension or blood pressure 140/90 mm Hg (1
mm Hg=0.133 kPa) [18]. Diabetes was defined as self-reported
physician diagnosis of diabetes or definite clinical records of
diabetes or fasting blood glucose ≥ 7.0 mmol/L [19].
Statistical Analysis
Participants’ baseline characteristics were presented as
means ± standard deviation (SD) for continuous variables and
numbers (percentages) for categorical variables. Comparison
of continuous variables between groups using the Student’s
t-test and chi-squared test for categorical variables. Hazard
ratios (HRs) with 95% confidence intervals (95% CIs) were
calculated to estimate the associations between HUA at
baseline with new-onset CKD by using Cox proportional
hazards regression models. None of the covariates were
adjusted for Model 1, covariates that were included in Model
2 were those that altered the hazard ratios for the effect of
CKD by more than 5% in and Analysis, and all of the covariates
were in the form of categorical variables. Finally, the covariates
included in Model 2 were age (<45 years, 45 years-64 years,
65 years), gender (male, female), BMI (<24.0 kg.m−2, 24.0
kg.m−2-27.9 kg.m−2, 28 kg.m−2), smoking status (non-smoker,
smoker, ex-smoker), drinking status (non-drinker, drinker, exdrinker),
diabetes (no, yes), hypertension (no, yes), TG ( 1.20
mmol/L, 1.21 mmol/L-2.00 mmol/L, 2.01 mmol/L) at baseline.
Stratified analyses were performed according to age, gender,
BMI, smoking and drinking status, occupation, diabetes, and
hypertension. Likelihood ratio tests were used to investigate
interactions.
SAS macro was used to examine the additive and multiplicative
interactions of CKD and related factors on the risk of HUA
[13]. relative excess risk due to interaction (RERI), attributable
proportion due to interaction, relative excess risk due to
interaction (RERI), attributable proportion due to interaction,
AP), synergy index (SI). Where, RERI represents the relative
risk attributable to additive interaction, AP represents the
proportion of the total effect attributable to additive interaction
when two factors are present at the same time, and SI
represents the ratio of the effect when two factors are present
at the same time to the sum of the independent effects of two
factors. If the confidence interval for RERI and AP does not
contain 0, and the confidence interval for SI does not contain
1, then there is an additive interaction [20,21]. All statistical
analyses were performed with SAS program, version 9.4 (SAS
Institute Inc., Cary, NC, USA) and R software (R Foundation for
Statistical Computing), version 4.3.2. All statistical tests were
two-sided, and P <0.05 was considered statistically significant.
Results
Baseline Characteristics of the Study Population
As shown in Table 1, a total of 28,422 subjects were included in the second part of the study, of which 61.70% were males, and the proportion of people <45 years old was the highest, reaching 52.13%. Except for occupation and diabetes, there were statistically significant differences in baseline characteristics between the patients with HUA and the non-HUA population. Among them, there were statistically significant differences in age, education level, smoking, drinking and physical exercise between HUA group and non-HUA group (all P<0.05). In addition, the proportion of men and hypertension in HUA group was higher than that in non-HUA group, and the difference was statistically significant (all P<0.05). The levels of BMI, SBP, DBP, FPG, TC, TG and LDL-C in HUA population were higher than those in non-HUA population. HDL-C was lower than that of non-HUA population, and the differences were statistically significant (all P<0.05).
Table 1: Baseline characteristics of study participants, stratified by HUA
| Variables |
Total population |
HUA |
Non-HUA |
P value |
| (N=28,422) |
(N=3869) |
(N=24,553) |
| Age (years), N (%) |
| <45 |
14,816 (52.13) |
2023 (52.29) |
12,793 (52.10) |
<0.001 |
| 45-64 |
10,833 (38.11) |
1407 (36.37) |
9426 (38.39) |
| ≥ 65 |
2773 (9.76) |
439 (11.34) |
2334 (9.51) |
| BMI (kg.m-2), N (%) |
| <24.0 |
15,407 (54.21) |
1677 (43.34) |
13,730 (55.92) |
<0.001 |
| 24.0-27.9 |
10,503 (36.95) |
1649 (42.62) |
8854 (36.06) |
| ≥ 28.0 |
2512 (8.84) |
543 (14.04) |
1969 (8.02) |
| Male, N (%) |
17,536 (61.70) |
3189 (82.42) |
14,347 (58.43) |
<0.001 |
| Education, N (%) |
| Junior high school or below |
11,103 (39.06) |
1381 (35.69) |
9722 (39.60) |
<0.001 |
| High school |
7776 (27.36) |
1077 (27.84) |
6699 (27.28) |
| Junior college |
5669 (19.95) |
812 (20.99) |
4857 (19.78) |
| Bachelor's degree or above |
3874 (13.63) |
599 (15.48) |
3275 (13.34) |
| Occupation, N (%) |
| Front-line worker |
21,983 (77.35) |
2961 (76.53) |
19,022 (77.47) |
0.193 |
| White-collar worker |
6439 (22.65) |
908 (23.47) |
5531 (22.53) |
| Smoking status, N (%) |
| Non-smoker |
15,459 (54.39) |
1534 (39.65) |
13,925 (56.71) |
<0.001 |
| Smoker |
10,565 (37.17) |
1889 (48.82) |
8676 (35.34) |
| Ex-smoker |
2398 (8.44) |
446 (11.53) |
1952 (7.95) |
| Drinking status, N (%) |
| Non-drinker |
21,128 (74.34) |
2375 (61.39) |
18,753 (76.38) |
<0.001 |
| Drinker |
6022 (21.19) |
1263 (32.64) |
4759 (19.38) |
| Ex-drinker |
1272 (4.47) |
231 (5.97) |
1041 (4.24) |
| Physical exercise, N (%) |
| No |
3854 (13.56) |
542 (14.01) |
3312 (13.49) |
0.008 |
| Occasionally |
11,233 (39.52) |
1601 (41.38) |
9632 (39.23) |
| Often |
13,335 (46.92) |
1726 (44.61) |
11,609 (47.28) |
| Diabetes, N (%) |
1947 (6.85) |
280 (7.24) |
1667 (6.79) |
0.306 |
| Hypertension, N (%) |
8286 (29.15) |
1604 (41.46) |
6682 (27.21) |
<0.001 |
| TC (mmol/L), Mean ± SD |
4.70 ± 0.89 |
4.88 ± 0.94 |
4.67 ± 0.88 |
<0.001 |
| TG (mmol/L), Mean ± SD |
1.50 (1.10, 2.30) |
2.10 (1.50, 3.10) |
1.50 (1.00, 2.10) |
<0.001 |
| HDL-C (mmol/L), Mean ± SD |
1.37 ± 0.35 |
1.25 ± 0.32 |
1.39 ± 0.35 |
<0.001 |
| LDL-C (mmol/L), Mean ± SD |
3.05 ± 0.74 |
3.14 ± 0.75 |
3.04 ± 0.73 |
<0.001 |
| Data were presented as means ± SD for continuous variables with normal distribution, M (P25, P75) for continuous variables with skewed distribution, and numbers (percentages) for categorical variables. Differences between groups were compared using the Student's t-test for continuous variables with normal distribution, Wilcoxon rank-sum test for continuous variables with skewed distribution, and Chi-squared test for categorical variables. HUA, hyperuricemia; BMI, body mass index. TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol |
Association between HUA and Risk of Newonset CKD
After adjusting for possible confounders, Cox regression showed an association between HUA, and the risk of developing CKD, with a 0.28-fold increase in the risk of developing CKD in the baseline HUA population compared with the non-HUA population (HR=1.28, 95% CI:1.12-1.47), as shown in Table 2.
Table 2: Associations between HUA at baseline with new-onset CKD
| |
N |
No of Events (%) |
Model 1 |
Model 2 |
| HR (95% CI) |
P value |
HR (95% CI) |
P value |
| Non-HUA |
24,553 |
933 (3.80%) |
1.00 (Ref) |
|
1.00 (Ref) |
|
| HUA |
3869 |
279 (7.21%) |
1.70 (1.48-1.93) |
<0.001 |
1.28 (1.12-1.47) |
<0.001 |
| Model 1 was not adjusted for any covariates. Model 2 was adjusted for age (<45 years, 45 years-64 years, ≥ 65 years), gender (male, female), BMI (<24.0 kg.m-2, 24.0 kg·m-2-27.9 kg·m-2, ≥ 28 kg.m-2), smoking status (non-smoker, smoker, ex-smoker), drinking status (non-drinker, drinker, ex-drinker), diabetes (no, yes), hypertension (no, yes), TG (≥ 1.20 mmol/L, 1.21 mmol/L-2.00 mmol/L, ≥ 2.01 mmol/L) at baseline. CKD, chronic kidney disease; HUA, hyperuricemia; HR, hazard ratio; CI, confidence interval. |
According to the baseline age of the study subjects (<45 years, 45-64 years, ≥ 65 years), sex (male, female), BMI (<24.0 kg.m−2, 24.0 kg.m−2-27.9 kg.m−2, ≥ 28.0 kg.m−2), smoking (no, yes, quit), and alcohol consumption (no, yes, quit), diabetes (no, yes) and hypertension (no, yes) were analysed in subgroups, and after adjusting for possible confounders, Cox regression showed that the association between HUA and the risk of developing CKD was more pronounced in the age ≥ 65 years, female, BMI <24 kg.m−2, quit smoking, workers, non-diabetic and hypertensive populations, with HR (95% CI) 1.63 (1.25-2.11), 1.55 (1.13-2.13), 1.37 (1.10-1.71), 1.54 (1.10-2.14), 1.30 (1.11-1.52), 1.31 (1.12- 1.53), and 1.45 (1.22-1.73), respectively. In addition, there was a multiplicative interaction between the associations of HUA and hypertension with the risk of developing CKD (P<0.05), and no multiplicative interactions were observed between the associations of HUA and other subgroups of categorical variables with the risk of developing CKD (all P>0.05) (Figure 1).
Analysis of the Combined Effect of HUA and Hypertension on the Risk of Developing CKD
As shown in Table 3, after adjusting for confounders, the risk of CKD in the baseline non-HUA and hypertensive, HUA and non-hypertensive, and HUA and hypertensive populations was 1.60 times (HR=1.60, 95% CI:1.39-1.83), 1.09 times (HR=1.09, 95% CI:0.87-1.37), 1.09 (HR=1.09, 95% CI:0.87-2.37), and 2.32 (HR=2.32, 95% CI:1.94-2.79) times.
Table 3: The joint effect of HUA and HTN on the risk of CKD
| |
HTN |
N |
HR (95% CI) |
P value |
| Non-HUA |
No |
17871 |
1 |
|
| Yes |
6682 |
1.60 (1.39-1.83) |
<0.001 |
| HUA |
No |
2265 |
1.09 (0.87-1.37) |
0.458 |
| Yes |
1604 |
2.32 (1.94-2.79) |
<0.001 |
| HRs (95% CI) was adjusted for age (<45 years, 45 years-64 years, ≥ 65 years), gender (male, female), BMI (<24.0 kg.m-2, 24.0 kg.m-2-27.9 kg.m-2, ≥ 28 kg.m-2), smoking status (non-smoker, smoker, ex-smoker), drinking status (non-drinker, drinker, ex-drinker), diabetes (no, yes), TG (≥1.20 mmol/L, 1.21 mol/L-2.00 mmol/L, ≥ 2.01 mmol/L) at baseline. CKD, chronic kidney disease; HUA, hyperuricemia; HTN, hypertension; HR, Hazard Ratio; CI, Confidence Interval. |
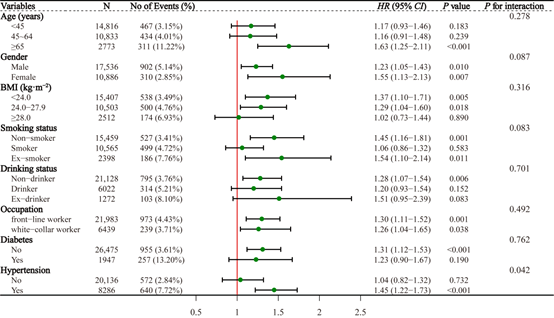
Figure 1: Stratified associations between HUA and new-onset CKD by age, gender, BMI, smoking status, drinking status, occupation, diabetes, and hypertension
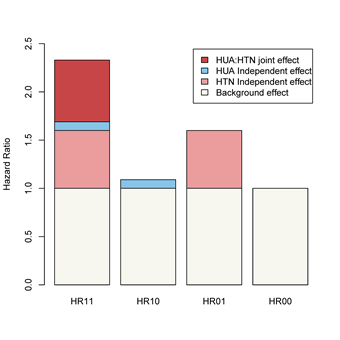
Figure 2: Schematic diagram of the interaction between HUA and hypertension on the risk of developing CKD
Analysis of the Interaction between HUA and Hypertension on the Risk of Developing CKD
As shown in Table 4, after adjusting for confounders, there was a positive multiplicative and additive interaction between HUA and hypertension on the risk of developing CKD, and the percentage of the additive interaction was 27%; the product term INTM (95% CI) of HUA and hypertension was 1.33 (1.01- 1.77); and the evaluators of the additive interaction, the RERI (95% CI), AP (95% CI), and SI (95% CI) were 0.64 (0.21-1.07), 0.27 (0.11-0.43), and 1.93 (1.16-3.19), respectively (Figure 2).
Table 4: Estimates of multiplicative and additive interaction of HUA and HTN on the risk of CKD
| |
Estimate |
95% CI |
P value |
| INTM |
1.33 |
1.01-1.77 |
0.042 |
| RERI |
0.64 |
0.21-1.07 |
0.004 |
| AP |
0.27 |
0.11-0.43 |
<0.001 |
| SI |
1.93 |
1.16-3.19 |
0.011 |
| Estimates was adjusted for age (<45 years, 45 years-64 years, ≥ 65 years), gender (male, female), BMI (<24.0 kg.m-2, 24.0 kg.m-2-27.9 kg.m-2, ≥ 28 kg.m-2), smoking status (non-smoker, smoker, ex-smoker), drinking status (non-drinker, drinker, ex-drinker), diabetes (no, yes), TG (≥ 1.20 mmol/L, 1.21 mmol/L-2.00 mmol/L, ≥ 2.01 mmol/L) at baseline. CKD, chronic kidney disease; HUA, hyperuricemia; HTN, hypertension; CI, confidence interval; INTM, interaction multiplicative; RERI, relative excess risk due to interaction; AP, attributable proportion due to interaction; SI, synergy index. |
Discussion
Studies have found that uric acid can lead to kidney injury by
forming urate crystals that obstruct renal tubules, inducing
proliferation of vascular smooth muscle cells and reduction of
endothelial NO [22,23], and activating the renin-angiotensinaldosterone
system to cause an increase in blood pressure;
furthermore, elevated levels of uric acid promote medial
thickening of small pre-glomerular arterioles, which is directly
correlated with glomerular capillary pressure [24]. Such
small arterial changes in the kidney may lead to ischaemia
and hypoxia, which are the strongest triggers of tubuleinterstitial
fibrosis [25]. Experimental studies have shown that
hyperuricaemia accelerates the deterioration of renal function
through high systemic blood pressure and cyclooxygenasemediated
thromboxane-induced vascular disease [26]. In
addition, renal inflammation occurring in the context of
hyperuricaemia plays a key role. Urate-induced stimulation
of NLRP3 inflammatory vesicles and release of interleukin-1
β promotes chemokine signalling in proximal tubular cells,
leading to tubular injury and proteinuria. Uric acid also induces Toll-like receptor-dependent activation of renal mesangial cells,
increases local expression of chemokines, promotes epithelial
mesenchymal transition in renal tubular cells by decreasing
E-calmodulin expression and increases fibronectin synthesis
by up-regulating lysyl oxidase in renal tubular epithelial cells
[27-30]. These alterations simultaneously promote intrarenal
inflammation, interstitial fibrosis, and CKD. These mechanisms
provide a biologically plausible explanation for the results of
the association between HUA and the risk of developing CKD
in this study. The results of this study showed that the risk of
CKD in the baseline HUA population was increased by 0.28-fold
compared with that in the non-HUA population (HR=1.28, 95%
CI:1.12-1.47). Therefore, the management of HUA and sUA
should be emphasised in the prevention and control of CKD,
and the active treatment of HUA and control of sUA levels are
beneficial in preventing the development of CKD as well as
delaying the decline of eGFR.
The results of a cohort study in the Netherlands showed
that the association between sUA and CKD and eGFR decline
was stronger in hypertensive populations compared to nonhypertensive
populations, and that there was a multiplicative
interaction between sUA and hypertension on both the
occurrence of CKD and the decline in eGFR [12]. However,
this study did not further investigate whether there was an
additive interaction between sUA and hypertension on the
development of CKD. In the present study, it was found that
there was not only a positive multiplicative interaction but also
a positive additive interaction between HUA and hypertension
on the development of CKD, with the percentage of additive
interaction being 27%. The existence of this interaction may
be due to the mutual influence of HUA and hypertension and
their promotion of each other, resulting in the study subjects
being more prone to develop CKD when HUA and hypertension
coexist. Studies based on the Gusau cohort have shown
that hyperuricaemia may contribute to the development of
hypertension by causing microalbuminuria [31,32]. Harrison
et al. proposed a secondary "strike" model to explain the
mechanism of uric acid-induced hypertension [33,34]. The first
"hit" is the activation of the renin-angiotensin-aldosterone
system and the inhibition of nitric oxide synthesis, which
promotes endothelial dysfunction, vascular smooth muscle
cell proliferation, and sodium reabsorption, leading to a
moderate but sustained increase in systemic blood pressure.
The second "hit" involves the immune system. Uric acid
released in response to hypertension-induced damage can be
recognised as a dangerous molecule by pattern recognition
receptors. Downstream signalling from these receptors leads
to dendritic cell maturation and activation of resting T-cells, but
it also triggers inflammatory vesicles and induces the secretion
of pro-inflammatory cytokines. This pro-inflammatory
environment simultaneously expands extracellular fluid volume
and increases vascular resistance, which further promotes
systemic hypertension. Correspondingly, hyperuricaemia is
also common in patients with essential hypertension and the
use of thiazide diuretics increases serum uric acid levels in
hypertensive patients [35]. The above mechanisms may partly
explain the fact that the effect of HUA and hypertension when
co-existing is greater than the effect of HUA alone, i.e., there
is a synergistic effect of HUA and hypertension on the risk of
developing CKD.
The study has a large sample size, collects relatively
comprehensive information, and adjusts for confounding
factors as much as possible in the regression analyses, making
the results highly credible. However, there are also some
shortcomings. Firstly, this study only included data from the
baseline and first follow-up surveys of the Jinchang cohort, and
the follow-up period was relatively short and limited to the
Jinchang cohort population, which restricted the extrapolation
of the results. Therefore, the results still need to be further
validated in a multi-centre, large-scale cohort. In addition,
as an observational study, although this study adjusted for
confounders as much as possible, it still could not avoid the
interference of residual confounding. Finally, considering that
the study population was already exposed to CKD risk factors
such as heavy metals at the time of the baseline investigation, a
quantitative assessment of metal exposure will help to further
elucidate the pathogenesis of CKD.
Conclusion
In summary, HUA is a risk factor for the development of CKD,
and there is a synergistic effect with hypertension on the
development of CKD. According to the results of this study,
for the early prevention and diagnosis of CKD, attention and
intervention should be focused on those with HUA combined
with hypertension, so that the limited health resources and
funds can be utilized to the greatest extent reasonably. In
addition, the results of this study will also help to further
explore the mechanism of HUA leading to the development of
CKD and provide new ideas for the scientific prevention and
treatment of CKD.
Author Contributions
Z.M. conceived and drafted the original manuscript. X.H., P.H.
analyzed the data and performed the statistical analysis. J.S.,
Y.W., Y.T., H.L., F.X. and M.N. collected and cleaned the data.
X.H. designed the analytic strategy and revised the manuscript
for intellectual content. All authors have read and agreed to
the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of
Gansu Province (20JR10RA599).
Institutional Review Board Statement
This study involving human participants was in accordance
with the Helsinki Declaration of 1964 and its later amendments
or comparable ethical standards. The Ethics Committee of the
School of Public Health of Lanzhou University approved this
study (Ethical Approved Code: 2015-01).
Informed Consent Statement
All participants signed informed consent.
Data Availability Statement
The data underlying this article cannot be shared publicly
due to data protection reasons. The data will be shared upon
reasonable request to the corresponding author.
Acknowledgement
We would like to sincerely thank all of the participants and
researchers from Jinchang Nonferrous Metal Company, the
Worker’s Hospital of JNMC, and Lanzhou University in the
Jinchang Cohort Study for their contributions and collaboration.
Conflict Of Interest
The authors declare that the research was conducted in the
absence of any commercial or financial relationships that could
be construed as potential conflicts of interest.
References
- Eknoyan G, Lameire N, Eckardt K, Kasiske B, Wheeler D, et al. (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney int. 3(1):5-14.
[Google Scholar]
- Bikbov B, Purcell C, Levey AS, Smith M, Abdoli A, et al. (2020) Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the global burden of disease Study 2017. Lancet. 395(10225):709-733.
[Crossref] [Google Scholar]
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, et al. (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease Study 2017. Lancet. 392(10159):1789-1858.
[Crossref] [Google Scholar]
- Zhang L, Wang F, Wang L, Wang W, Liu B, et al. (2012) Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet. 379(9818):815-822.
[Crossref] [Google Scholar]
- Wang L, Xu X, Zhang M, Hu C, Zhang X, et al. (2023) Prevalence of chronic kidney disease in China: Results from the sixth china chronic disease and risk factor surveillance. JAMA Intern Med. 183(4):298-310
[Crossref] [Google Scholar]
- Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V (2021) Chronic kidney disease. Lancet. 398(10302):786-802.
[Crossref] [Google Scholar]
- Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, et al. (2018) Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: Report of a scientific workshop organized by the national kidney foundation. Am J Kidney Dis. 71(6):851-865.
[Crossref] [Google Scholar]
- Chen WY, Fu YP, Zhou M (2022) The bidirectional relationship between metabolic syndrome and hyperuricemia in China: A longitudinal study from CHARLS. Endocrine. 76(1):62-69.
[Crossref] [Google Scholar]
- Qiu Y, Zhao Q, Wang N, Cui SH, Yu YT, et al. (2021) Association of hyperuricemia with risk of incident chronic kidney disease in adult in Songjiang district, Shanghai: A follow-up study. Zhonghua Liu Xing Bing Xue Za Zhi. 42(9):1607-1614.
[Crossref] [Google Scholar]
- Zhenwei S (2017) Chronic kidney disease prediction models based on large-scale health management cohort data.
- Chen JH, Tsai CC, Liu YH, Wu PY, Huang JC, et al. (2022) Sex difference in the associations among hyperuricemia with new-onset chronic kidney disease in a large Taiwanese population follow-up study. Nutrients. 14(18):3832.
[Crossref] [Google Scholar]
- Sedaghat S, Hoorn EJ, Van Rooij FJ, Hofman A, Franco OH, et al. (2013) Serum uric acid and chronic kidney disease: The role of hypertension. PLoS One. 8(11):e76827.
[Crossref] [Google Scholar]
- Nie ZQ, Ou YQ, Zhuang J, Qu YJ, Mai JZ, et al. (2016) Application of SAS macro to evaluated multiplicative and additive interaction in logistic and Cox regression in clinical practices. 37(5):737-740.
[Crossref] [Google Scholar]
- Bai Y, Yang A, Pu H, Dai M, Cheng N, et al. (2017) Cohort profile: The China metal-exposed workers cohort study (jinchang cohort). Int J Epidemiol. 46(4):1095-1096.
[Crossref] [Google Scholar]
- Bai Y, Yang J, Cheng Z, Zhang D, Wang R, et al. (2022) Cohort profile update: The China metal-exposed workers cohort study (jinchang cohort). Eur J Epidemiol. 37(6):641-649.
[Crossref] [Google Scholar]
- Bai Y, Pu H, Dai M, Cheng N, Li H, et al. (2015) Study progress on China Jinchang Cohort. Lan Uni J (Medical Edition), 41(06):29-33.
[Crossref]
- Levey AS, Stevens LA, Schmid C, Zhang YP, Castro AF, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med. 150(9):604-612.
[Crossref] [Google Scholar]
- 2018 Chinese guidelines for prevention and treatment of hypertension-a report of the revision committee of Chinese guidelines for prevention and treatment of hypertension.
- Wang H (2020) Several issues in interaction analysis and type I error control. Shanxi Medical University.
- Wang H, Gao X, Gao Q, Li Y, He S, et al. (2020) Review of interactions in medical research. Chin Heal Stat. 37(4):629-636.
[Google Scholar]
- Mulay SR, Shi C, Ma X, Anders HJ (2018) Novel insights into crystal-induced kidney injury. Kidney Dis. 4(2):49-57.
[Crossref] [Google Scholar]
- Xu C, Lu A, Lu X, Zhang L, Fang H, et al. (2017) Activation of renal (pro) renin receptor contributes to high fructose-induced salt sensitivity. Hypertension. 69(2):339-348.
[Crossref] [Google Scholar]
- Sanchez-Lozada LG, Tapia E, Avila-Casado C, Soto V, Franco M, et al. (2002) Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol Renal Physiol. 283(5):F1105-F1110.
[Crossref] [Google Scholar]
- Liu MN, Ning XX, Li R, Yang Z, Yang XX, et al. (2017) Signalling pathways involved in hypoxia-induced renal fibrosis. J Cell Mol Med. 21(7):1248-1259.
[Crossref] [Google Scholar]
- Kang DH, Nakagawa T, Feng LL, Watanabe S, Han L, et al. (2002) A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 13(12):2888-2897.
[Crossref] [Google Scholar]
- Crisan TO, Cleophas MCP, Oosting M, Lemmers H, Toenhake-Dijkstra H, et al. (2016) Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann Rheum Dis. 75(4):755-762.
[Crossref] [Google Scholar]
- Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, et al. (2013) Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol. 304(5):F471-F480.
[Crossref] [Google Scholar]
- Xiao J, Fu C, Zhang X, Zhu D, Chen W, et al. (2015) Soluble monosodium urate, but not its crystal, induces toll like receptor 4-dependent immune activation in renal mesangial cells. Mol Immunol. 66(2):310-318.
[Crossref] [Google Scholar]
- Zhou Y, Wang XH, Jiang L, Tan RY, Xiong M, et al. (2010) Uric acid increases fibronectin synthesis through upregulation of lysyl oxidase expression in rat renal tubular epithelial cells. Am J Physiol Renal Physiol. 299(2):F336-F346.
[Crossref] [Google Scholar]
- Jiang YB, Yu J, Zhang Q, Ren LY, He Y, et al. (2020) Microalbuminuria mediates the association between serum uric acid and elevation of blood pressure: A longitudinal analysis in the Gusu cohort. J Hypertens. 38(4):625-632.
[Crossref] [Google Scholar]
- Jiang Y (2020) A longitudinal study of hyperuricemia, microalbuminuria and the risk of hypertension. Suzhou University.
[Crossref]
- Harrison DG, Vinh A, Lob H, Madhur MS (2010) Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 10(2):203-207.
[Crossref] [Google Scholar]
- Ponticelli C, Podesta MA, Moroni G (2020) Hyperuricemia as a trigger of immune response in hypertension and chronic kidney disease. Kidney Int. 98(5):1149-1159.
[Crossref] [Google Scholar]
- Sanchez-Lozada LG, Rodriguez-Iturbe B, Kelley EE, Nakagawa T, Madero M, et al. (2020) Uric acid and hypertension: An update with recommendations. American Journal of Hypertension, 33(7):583-594.
[Crossref] [Google Scholar]
- Bai Y, Pu H, Dai M, Cheng N, Li H, et al. (2015) Progress of China jinchang cohort study. Lan Uni J (Medical Edition). 41(06):29-33+38.
[Crossref]
Citation: Ma Z, Hu X, Hu P, Shi J, Wang Y, et al. (2023) Multiplicative and Additive Interactions of Hyperuricemia and Hypertension on the Risk of Chronic Kidney Disease: Evidence from a Prospective Population-based Cohort Study. J Healthc Commun. 8:8060.
Copyright: © 2023 Ma Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.