Research Article - (2024) Volume 8, Issue 2
Nalbuphine Nasal Spray: Analgesic Efficacy and Safety
Igor E. Kuznetsov1* and
Volodymyr G. Tymko2
1Department of Pharmacy, Clinical and Diagnostic Center Pharmbiotest, Kyiv, Ukraine
2Department of Pharmacy, Pharmaceutical Company Microkhim, Kyiv, Ukraine
*Correspondence:
Igor E. Kuznetsov, Department of Pharmacy, Clinical and Diagnostic Center Pharmbiotest, Kyiv,
Ukraine,
Email:
Received: 24-Jun-2024, Manuscript No. IPIPR-23-20492;
Editor assigned: 27-Jun-2024, Pre QC No. IPIPR-23-20492 (PQ);
Reviewed: 11-Jul-2024, QC No. IPIPR-23-20492;
Revised: 18-Jul-2024, Manuscript No. IPIPR-23-20492 (R);
Published:
15-Aug-2024, DOI: 10.21767/ipipr.8.02.011
Abstract
Opioids are used for the treatment of moderate to severe pain that is not responsive to other
analgesics. Powerful opioid analgesics (full mu-agonists), such as morphine and fentanyl, are highly
effective but have multiple harmful side effects, including abuse and dependence. The decades-long
search for an ‘ideal analgesic’ that provides fast pain relief for various types of pain, has long-lasting
effects, is well-tolerated and can be taken orally has led to the development of biased opioid agonists
providing potent pain relief with reduced side effects. These promising new medications still need
extensive clinical investigation, while the potential for pharmacokinetic improvements of well-studied
and long-used opioid medications remains.
Nalbuphine, a mixed partial mu-receptor antagonist and kappa-receptor agonist, is as effective as
morphine in relieving moderate to severe pain and has no serious side effects. It could be considered
close to an 'ideal analgesic', with one exception of being administered solely through parenteral
routes due to extensive pre-systemic metabolism and poor oral bioavailability.
Intranasal nalbuphine delivery represents a safe and non-invasive alternative to parenteral routes
of administration. Although nalbuphine has been used clinically for 40 years, the first results of
clinical trials on nasal administration of the injectable solution were published in 2019. Certain
progress has been made in the pharmaceutical development of nasal forms of nalbuphine, leading
to the recent development of a nasal spray. Retrospective analysis of the issue and recently
published data from clinical investigations of the newly developed nalbuphine nasal spray, are briefly
reviewed.
Keywords
Nalbuphine nasal spray; Pain management; Non inferiority clinical trial; Patient-controlled
analgesia
Introduction
Pain is not only a common symptom of a range of etiologies
but also a pathogenetic factor in the transition from acute
to chronic pain [1,2].
A variety of clinical protocols and guidelines have been
developed for effective pain management in speci ic
patient populations, taking into consideration the origin,
character and topology of pain, as well as possible side
effects and complications.
A common feature of these recommendations is the use of
opioid analgesics to cope with moderate or severe pain
that is resistant to non-opioid and adjuvant analgesics.
For many patients with severe pain, opioids are the only
avenue for analgesia [3-5]. Historically, the opium poppy was
used as an analgesic back in ancient Greece and
opium alkaloid morphine has been used as a pain reliever
since the early 1800’s. More than 150 years later, binding
sites for opioids were identified using radiolabeled
ligands and based on physiological and pharmacological
data; these structures were classified into four main types,
namely mu, delta, kappa and nociception opioid receptors
[5,6].
The era of synthetic opioids began parallel to the
differentiation of opioid receptor types and led to the
development of the majority of opioid medications currently
used. In the past decade, a variety of biased opioid agonists
have been designed using structure based methodology.
These next generation medicines are highly promising but still
need extensive clinical investigations. The purpose of this
brief review is to highlight the potential of improving the
pharmacokinetic properties and changing the route of
administration of currently used opioid drugs, which could
enhance pain management in a wide range of patients in
outpatient and home settings shortly.
Opioid Receptors’ Origin and Effects
Mu, delta and kappa Opioid Receptors (ORs) mediate
analgesia but have differing side effects, likely due to the
variable regional expression, plasticity and functional activity
of receptors in different parts of central and peripheral organ
systems. Opioid drugs such as morphine, codeine,
methadone, fentanyl and their derivatives are primarily muagonists
that have the most potent analgesic effect and the
highest side effects. Kapa-agonists also have high analgesic
efficacy but less expressed adverse reactions that raised
hopes that selective kappa-agonists would provide analgesia
without the side effects of morphine-like mu-opioids such as
addiction, respiratory depression, constipation and urinary
retention. Therefore, selective kappa-agonists came into the
focus of efforts, but soon it was found they cause
psychotomimetic, dysphoria, sedation, diuresis and
constipation with weaker analgesic effects than mu-opioids.
The strategy of increasing the selectivity of ligands for ORs to
maximize therapeutic over side effects has proven elusive [7].
The ORs that mediate the analgesic effects of endogenous
and exogenous opioid agents belong to the seventransmembrane
type A (rhodopsin-like) Guanine nucleotidebinding
Protein-Coupled Receptor (GPCR) family. These
receptors are encoded by individual genes located on
separate chromosomes and exhibit 50%-70% sequence
homology in extant vertebrates. ORs encoding genes
originated from a duplication of the ancestral opioid
unireceptor gene and subsequent divergent adaptive
evolution. Each type of ORs shares similar composition and
properties and activates the inhibitory G-protein cascade but
is distributed differently in central and peripheral nervous
structures and other tissues demonstrating slightly different
qualities and variability in the opioid response [8-10].
Effector Mechanism and Signal Transmission
Ligand binding in the pocket formed by transmembrane
domain of the receptor leads to conformational changes of
the intracellular C-terminus of the receptor that allows GPCR
coupling to the heterotrimeric G-protein that activates
intracellular signal transduction via GDP to GTP substitution at
the Gα subunit and dissociation of Gα and Gβγ subunits to
launch G-protein intracellular signaling cascades. The opioid
receptors are almost exclusively inhibitory, interacting
primarily through the Giα and Goα proteins of Gi/o family. The
Gα subunit inhibits adenylyl cyclases and cAMP production,
whereas Gβγ complex directly interacts with different ion
channels. Mu, Delta and Kappa Opioid Receptors (MOR, DOR
and KOR) can modulate pre-and postsynaptic Ca2+ channels,
suppress Ca2+ influx, activate G-protein-coupled Inwardly
Rectifying K+ (GIRK) channels, inhibits Na+ channels in the
dorsal root ganglia neurons and glutamate excitatory
postsynaptic currents in the spinal cord neurons. These
processes cause attenuation of neurons excitability and
transmission of nociceptive impulses at all neuraxis levels and
the suppression of pronociceptive neuropeptides release
resulting in reduced pain perception [11,12].
ORs interact with such intracellular signal transducers as Gproteins,
arrestins and/or GPCR kinases, functioning as part of
a three component system of receptor-transducer effector
where each component poses a range of genetic variants with
different functional performances. The activation of these
transducers triggers non-overlapping signaling pathways that
determine the ligand-specific responses. Given the four major
types of ORs, the eight isoforms of Gi/o transducers and the
pools of their splice variants, it still is unclear how an
extracellular ligand exerts a specific intracellular effect by
coupling the receptor with a proper transducer [13-15]. One
OR can couple to more than one Gi/o protein isoforms due to
their high structural similarity, resulting in different coupling
efficiencies in certain GPCR-G protein pairs. Such pairs are
characterized by higher or lower binding kinetics and take
specific conformations that determine the preferential
coupling of certain transducers. The type of selected
transducer determines the signaling cascade to launch inside
the cell. The concept of functional selectivity (biased agonism)
the ligand dependent selection of certain signal transduction
pathways resulting in specific cellular effects is the basis for
structure-based drug design [16,17].
Biased Agonism
The recognition that different agonists binding to the same
receptor can produce varying effects has led to a revision of
the two state model of receptor signaling as on/off switches
and to the promotion of the concept of “biased agonism”,
implying functional selectivity and ligand-directed signaling.
Compared to endogenous “balanced” agonists, which activate
different G-proteins and β-arrestins equally, functionally
selective “biased” agonists can selectively activate G-proteins
while blocking β-arrestins or vice versa [18]. β-arrestins
represent a family of multifunctional cytoplasmic proteins that
not only regulate nearly all aspects of GPCR activity, including
desensitization, downregulation, trafficking and signaling via binding to the activated receptors, but also couple to
numerous members of signaling cascades, including the
mitogen activated protein kinases, the serine/threonine and
the tyrosine kinases, nuclear factor-κB and phosphoinositide
3-kinase, acting as adaptors and scaffolds.
The commonly used opioid drugs, such as morphine, codeine,
methadone and fentanyl are MOR agonists that induce
analgesia through Gαi pathway signaling. However, their side
effects are mediated via β-arrestin pathway signaling
downstream of MOR activation. The analgesic action of the
KOR agonists is mediated by Gβγ subunit, while the adverse
effects are related to β-arrestin mediated activation of p38
MAPK, which regulates serotonin transporter and inward
rectifying potassium channel function in neurons of reward
processing centers (the dorsal raphe nucleus and ventral
tegmental area). Recently developed G-protein-biased MOR
and KOR agonists (Oliceridine, TRV734, PZM21) and (Triazole
1.1, RB-64), which display limited β-arrestin recruitment and
provide analgesia with fewer side effects compared with
morphine, is considered the therapeutic promising as optimal
opioid analgesics. On the other hand, it was shown that most
of the therapeutic and adverse effects of agonist-induced OR
activation are mediated by the G protein-dependent signaling
pathway and that many drugs described as G-protein-biased
agonists are actually low-intrinsic-efficacy agonists, which are
correlated with partial agonism rather than biased signaling
perse.
Functional parallelism between newly developed G-proteinbiased
ligands and partial opioid agonists or mixed agonists/
antagonists conceptually aligns with a recent trend in clinical
practice to utilize partial instead of full opioid agonists. The
mixed partial opioid agonists/antagonists must occupy a
greater fraction of the available pool of functional receptors
than full agonists to induce the equivalent analgesic response,
whereas acting as antagonists of the same or another type of
ORs; they typically exhibit reduced harmful adverse reactions
due to ceiling effect.
The Mixed Partial Opioid Agonists/Antagonists
The mixed partial agonists/antagonists comprise a chemically
heterogeneous group of synthetic and semi-synthetic opioids
that are widely used in clinical practice. Just three members
of this group buprenorphine (a partial MOR/NOR agonist and
a KOR/DOR antagonist), nalbuphine and similarly
butorphanol, which are combined MOR antagonists and KOR
partial agonists, are commonly used medications today. The
clinical advantages and limitations of mixed agonists/
antagonists are determined by three general features: (i) they
target multiple types of opioid receptors, (ii) produce low
intrinsic activity of opioid receptors after binding, resulting in
dose response curves exhibiting a ceiling effect at less than
the maximal effect produced by a full agonist and (iii) they
undergo extensive first pass metabolism. Poor oral
bioavailability (5%-17%) due to extensive pre-systemic
elimination determines the primarily injectable routes of
administration and hampers their application in oral dosage
forms needed for outpatient use.
Clinical availability of only injectable solutions significantly
limits the use of mixed agonists/antagonists outside of
hospital settings with few exemptions of transdermal
and buccal forms of buprenorphine and nasal forms of
butorphanol.
Material and Methods
Nalbuphine
Nalbuphine, a phenanthrene opioid derivative, structurally
close to naloxone (competitive ORs antagonist) and
oxymorphone (strong mu-agonist), has unique
pharmacological properties compared to other members of
the mixed agonists/antagonists group. In clinical practice,
nalbuphine is considered equianalgesic to morphine,
demonstrating an analgesic potency of 0.8-0.9 compared to
equimolar doses of morphine. Nalbuphine is a partial KOR
agonist that produces potent analgesic effects without the
harmful side effects associated with MOR activation and has
low addiction potential, making it the only opioid analgesic
not included in the controlled substances act.
As a weak MOR antagonist rather than an inverse agonist, it is
less likely to cause withdrawal when combined with other
opioids in acute pain management. In the general population,
withdrawal symptoms from nalbuphine are almost
nonexistent and its abuse potential is lower than the muagonists,
however, nalbuphine can be abused in certain
patient populations who are tolerant to potent opioids. The
ceiling effect of nalbuphine for respiratory depression
provides an important safety factor. Its depressive effects on
respiration plateau at a low dose and breathing is not further
compromised with higher doses of the drug. In low doses,
nalbuphine can reverse opioid related respiratory depression,
urinary retention and opioid induced pruritus caused by muagonists
without reversing analgesia. In contrast to muagonists,
nalbuphine does not cause pruritis even at high
doses because of its MOR antagonistic activity and lack of
histamine release. Nalbuphine at high doses does not affect
hemodynamics, cause hypotension, reduce cardiac output or
prolong the QTc interval, providing satisfactory analgesia for
most patients with acute myocardial infarction. Nalbuphine is
applied in patients with myocardial ischemia, especially in the
course of cardioprotective therapies. It is considered the
preferred opioid for patients with cardiovascular disease and
an excellent analgesic for intensive care patients receiving
vasopressor medications. Compared to mu-opioids,
nalbuphine exerts a less pronounced effect on the
gastrointestinal tract, where ORs are widely distributed. It
does not cause biliary spasms or colic and blocks the harmful
effects of potent opioids on gastrointestinal mobility, resulting
in reduced ileus. Nalbuphine therapy is associated with a
significantly lower incidence of constipation compared to
morphine. Since the sensation of bladder fullness is decreased
by MOR and DOR agonists (but not KOR) through the
inhibition of parasympathetic nerves, nalbuphine
administration was shown to improve urine output in patients
with opioid associated urinary retention.
In comparison to potent opioids like morphine, nalbuphine
itself causes little to no urinary retention.
Nalbuphine Pharmacokinetics
In addition to its pharmacological characteristics, nalbuphine
exhibits specific physicochemical and pharmacokinetic
properties that distinguish it from other mixed agonist/
antagonist opioids. The bioavailability of oral nalbuphine is
12%-17% due to extensive first pass metabolism, resulting in
the exclusive clinical use of injectable forms. In this regard,
physicochemical parameters such as the dissociation constant
(pKa), lipid solubility and protein binding, which determine
intestinal absorption, seem clinically less important than drug
metabolism and the pharmacological activity of the
metabolites. In contrast to buprenorphine and butorphanol,
which undergo cytochrome P450 oxidation, nalbuphine is
metabolized mainly by UDP glucuronosyltransferases
(UGT2B7, UGT1A3, UGT1A9) to inactive glucuronide
conjugates, resulting in fewer drug interactions and less
variable pharmacodynamics. Recent publications have
reported that nalbuphine glucuronides, especially
nalbuphine-6-glucuronide, have analgesic effects. The major
route of elimination is fecal, with little renal elimination. The
elimination half-life of nalbuphine is 2 to 5 hours, which
correlates with a duration of analgesic effect ranging from 3
to 6 hours. Systemic clearance of nalbuphine is reduced in
neonates (due to an immature enzyme system), elderly
individuals and patients with hepatic or renal insufficiency. As
a lipophilic small molecule, nalbuphine has a large volume of
distribution and readily crosses the blood brain barrier.
Nalbuphine Medical Use
Nalbuphine was first introduced into clinical practice in June
1979. Naturally, the following clinical trials aimed to compare
nalbuphine with other opioid analgesics, primarily morphine
(considered the gold standard), to determine its applicability,
efficacy and safety in controlling pain of different origins. In
1983, the first review of such studies discussed the results of
nine double blind clinical trials comparing morphine and
nalbuphine. Thirty years later, a meta-analysis of 15
randomized controlled trials, comparing nalbuphine with
morphine for analgesic effect and safety, showed no
significant difference between the two drugs in pain relief
with the pooled relative risk of 1.01 (95% CI, 0.91 to 1.11;
P=0.90). The incidence of opioid-associated side effects
(pruritus, nausea and vomiting, respiratory depression) was
significantly lower in patients receiving nalbuphine compared
with the morphine group. The conclusion of comparable
analgesic efficacy of morphine and nalbuphine with a better
safety profile of the latter was based on clinical data of 820
patients from North America, Europe and Asia who
experienced severe pain syndrome associated with
arthroscopic and otolaryngology surgery, hip replacement,
gynecology-related conditions and burn debridement pain.
Nalbuphine has been one of the most commonly used
analgesics for children since the 1980s due to its potent pain
relieving properties and favorable side effects profile (ceiling
effect for respiratory depression, minor urinary retention and minimal impact on hemodynamics) making it a safe option for
pediatric pain management.
Conceptually, nalbuphine seems to be a compound with the
qualities of an "ideal analgesic." It retains the analgesic
potency of morphine (the gold standard) while reducing side
effects. However, it still causes tolerance and addiction
development associated with chronic intake, although
nalbuphine has lower abuse potential compared to its
counterparts. Nalbuphine may induce opioid withdrawal
symptoms if administered to individuals tolerant to potent
opioids. In practical terms, the main limitation of nalbuphine is
its poor oral bioavailability, which necessitates administration
via injectable solutions intravenously, intramuscularly,
subcutaneously or rarely intrathecally.
Rectal Nalbuphine Administration
The availability of only injectable solutions greatly limits the
use of this effective and safe pain reliever outside of hospital
settings due to the lack of trained medical personnel. It is even
more disappointing since nalbuphine is a non-scheduled
medication that is easily accessible to patients with chronic
pain who are in home settings. This limitation became evident
over 40 years before, coinciding with the onset of clinical use
of nalbuphine. Animal and clinical studies have evaluated the
potential of bypassing hepatic first pass metabolism through
rectal and nasal administration of nalbuphine since the early
1980’s. Several groups of French researchers presented the
results of studies evaluating the analgesic efficacy, safety and
pharmacokinetics of rectally administered nalbuphine solution
in children undergoing general anesthesia for surgery. A
commercially available solution of nalbuphine hydrochloride
(10 mg/mL) was diluted with saline to a concentration of 2
mg/mL and administered rectally via a catheter at a dosage of
0.3 mg/kg (2.1 ml-3.2 ml based on body weight). It was shown
that rectal nalbuphine is rapidly absorbed (mean Tmax=25 ± 11
min) and provides adequate analgesia but is characterized by
highly variable pharmacokinetics (CV for Cmax and AUC equaled
62% and 68%, respectively). The absolute bioavailability of
rectal nalbuphine has not been experimentally determined
due to the study design; however, the authors inferred that it
is "better" when compared with published data on oral
nalbuphine.
Nasal Nalbuphine Administration
The intranasal route has garnered more attention due to its
potential to achieve bioavailability comparable to injectable
forms and provide convenient administration. In 1985, the
first monograph on the fundamentals and developmental
concepts of these medications was published summarizing
the advantages of intranasal drug delivery as: (i) bypassing
"first-pass" metabolism, (ii) efficient absorption into the
bloodstream through the highly vascularized microvillus
structured nasal mucosa and (iii) similar kinetics of systemic
delivery compared to parenteral administration for some
compounds. The first reports of therapeutic and
Pharmacokinetic (PK) studies of intranasally administered
opioids, buprenorphine to healthy volunteers and sufentanil to surgery patients, were presented by independent research
teams four years later, in 1989. It was reported that both
compounds were rapidly and effectively absorbed from the
nasal mucosa. The absolute bioavailability was 48.2% for
buprenorphine and 78.0% for sufentanil. There were no
significant differences in sedation between patients receiving
sufentanil intravenously and intranasally. Both medications
did not cause clinically important adverse reactions. The
authors concluded that the intranasal route of buprenorphine
and sufentanil administration may be an attractive alternative
to intravenous or intramuscular injection. In the following two
decades, a range of complementary strategies were
developed to enhance the bioavailability of intranasally
administered drugs, which included facilitating permeability,
preventing enzymatic degradation, inhibiting efflux
transporters and reducing mucociliary clearance. Plenty of
clinical trials have been conducted on the intranasal
administration of almost all opiate compounds used in
medical practice. The obtained results significantly expanded
the understanding of the impact of physicochemical
properties, physiological factors and pharmaceutical
procedures on the observed variations in absorption and
disposition of intranasal formulations. Finally, several
innovative nasal opioid medications have been developed and
approved for clinical use.
Despite decades of nalbuphine use in pain management and
the clear need for a nasal nalbuphine medication, the results
of the first human study on intranasal nalbuphine were only
published in 2019, while a brief mention of nasal nalbuphine
administration to children for perioperative analgesia
appeared in 2014. The first study reporting clinical usage of
intranasal nalbuphine was performed in the university
children’s hospital Zurich emergency department between
2017 and 2018. Infants aged 1-3 months with fever, not
requiring a partial or full sepsis work-up, were included in the
study. The study aims to evaluate the pharmacokinetics, pain
control and tolerability of a single intravenous (0.05 mg/kg)
and intranasal (0.1 mg/kg) administration of 10 mg/ml
nalbuphine solution for injection (OrPha Swiss, Switzerland).
The intranasal dosage was doubled because the expected
bioavailability of intranasal nalbuphine, calculated based on
lipophilicity and molecular weight, was anticipated to be
between 50%-80%. One milliliter syringe was utilized to
administer 25-200 microliters of nalbuphine solution through
the porous nozzle (MAD 300 Teleflex, USA). Patients were
assigned to two parallel groups using the open procedure,
which involved alternating to balance the bias in the numbers
of patients receiving nalbuphine solution either intranasally or
intravenously before each painful intervention (such as
establishing venous access, urinary catheterization and
lumbar puncture). Pain control and tolerability were assessed
using the Neonatal Infant Pain Scale (NIPS) for each
intervention. Adverse Events (AEs) and vital signs (oxygen
saturation, heart rate and blood pressure) were recorded at
baseline and during each intervention. In total, data from 52
infants, who received nalbuphine (26 intravenously and 26
intranasally), were collected for analysis of pain control, safety
and tolerability.
The analgesic effect of intranasal nalbuphine was found to be
similar to intravenous administration, with 67% and 71% of
cases reporting mild to no pain (NIPS<3), respectively. PK
analysis revealed similar exposure coverage following a single
administration of 0.1 mg/kg of nalbuphine intranasally and
0.05 mg/kg intravenously, suggesting an intranasal
bioavailability is close to 50% (41% (95% CI: 26%-56%)). The
authors mentioned that between 45% and 82% of patient’s
experienced severe pain during urinary catheterization and
lumbar puncture. Based on the exposure pain response
simulation, they suggested that increasing the intranasal
nalbuphine doses to 0.4 mg/kg may be necessary to achieve
pain control similar to that of an intravenous dose of 0.1 mg/
kg-0.2 mg/kg. The results of the first clinical study comparing
nalbuphine pharmacokinetics, analgesic efficacy, safety and
tolerability after intranasal and intravenous administration led
the authors to conclude that intranasal administration of
nalbuphine solution is a safe, non-invasive alternative
approach to the parenteral administration of nalbuphine. The
authors concluded that this approach can reduce pain for
pediatric patients and alleviate stress for parents and medical
staff.
The first clinical observation of intranasal nalbuphine
administration to adults was also conducted in Switzerland in
2017-2020. This observational cohort study aimed to analyze
data from trauma victims receiving analgesia by intranasal
nalbuphine administration in the prehospital phase. Trained
first responders enrolled patients according to the study
instructions; the inclusion of patients was non-consecutive
and there was no reference group in this study.
Administration of nalbuphine according to an algorithm was
required to assure patient safety and improve overall
treatment. Nalbuphine hydrochloride solution for injection 10
mg/ml (OrPha Swiss, Switzerland) was administered
intranasally using a syringe equipped with the porous nozzle
(MAD 300, Teleflex, USA) as described above. The dosage was
based on the patient's body weight, with 5 mg for adolescents
weighing 20 kg-44 kg and a maximum of 20 mg for adults
weighing over 75 kg with severe pain. The volume of the
solution administered in each nostril did not exceed 1 ml. The
risk of respiratory depression was monitored by pulse
oximetry and respiratory. Pain intensity was defined as a score
of 5 or higher on a Numeric Rating Scale (NRS), with 0
indicating no pain and 10 indicating the worst imaginable pain.
Nalbuphine should not be administered in cases of
altered consciousness, head trauma, alcohol consumption or
abnormal vital signs. Also, contraindications included known
allergy to the drug or its additives, patient refusal or body
weight less than 20 kg.
Data from 267 patients with extremity injuries and traumas to
the shoulder, knee, lower leg, trunk, thorax and abdomen
were analyzed statistically. The mean baseline pain intensity
in trauma victims assessed by first responders was 8
NRS points (IQR 7 to 9).
After intranasal administration of nalbuphine solution, most
patients experienced pain relief without any major adverse
events. Intranasal nalbuphine administration resulted in a
statistically significant and clinically relevant reduction in pain
levels, with a median decrease of 3 NRS units, being more
effective in adolescents than in patients aged 20 to 60 years.
Referring to the literature, obtained pain reduction of more
than 2 NRS points was deemed good pain relief. An average
pain reduction of 3 points on the NRS was also observed after
nasal administration of fentanyl in the pre-hospital phase. Of
the 267 trauma victims who received intranasal nalbuphine
solution, 145 (54.3%) experienced clinically relevant pain
reduction and 41 (15.3%) expressed dissatisfaction with the
treatment. The authors concluded that administering
nalbuphine nasally to acutely injured patients in the
prehospital setting is a potentially safe and effective
noninvasive pain management approach and a viable
alternative to parenteral administration.
Both research teams reached similar conclusions about the
clinical prospects of intranasal nalbuphine, despite substantial
differences between these clinical trials and specific
limitations associated with the study protocols. They also
encountered the same difficulties related to the nasal
administration of the injection solution. The point is the low
nalbuphine concentration of the licensed solution for injection
(10 mg/ml) and the limited volume of the nasal cavity. The
latter is the reason for the recommendations to reduce the
delivered unit volume to 100 mcl per nostril. An oversized unit
volume applied in the nasal cavity leads to greater surface
area deposition, causing swallowing or leakage of the
administered solution. Thus, to deliver a single therapeutic
dose of nalbuphine (10 mg-20 mg, i.e., 1.0 ml-2.0 ml of 10 mg/
ml solution), some consecutive units should be administered
at several minutes intervals. An effective approach is to use a
highly concentrated solution near the water solubility limit of
nalbuphine hydrochloride (35.5 mg/ml). However, this
approach presents challenges due to the poor stability of the
concentrated nalbuphine solution caused by rapid oxidation
following contact with atmospheric air and impurity
formation. Furthermore, nalbuphine tends to precipitate in
concentrated solutions at low temperatures. Early
pharmaceutical development of a metered nasal form of
nalbuphine was supposedly hindered by the poor stability of
the finished product.
Nalbuphine Nasal Spray
The pharmaceutical company Microkhim (Kyiv, Ukraine)
has recently realized a practical solution to the poor stability
issue and has developed a nalbuphine nasal spray
Apain®. The developers utilized the so called binary approach
to neutralize nalbuphine oxidation in an aqueous solution
by separating the dry formulation from the solvent. For
this purpose, an innovative spray bottle containing two
chambers separated by a destructible membrane and
equipped with a precise dosing pump has been developed.
A ready to use solution of nalbuphine hydrochloride
forms within two minutes after cranking the safety ring
on the spray bottle before the first use of the nasal spray.
Changing the position of the safety ring ruptures the
membrane between chambers, allowing dry ingredients
to dissolve.
The spray composition is ready for application when it turns
light blue. A pink color appears over time, indicating
impurities formation due to prolonged exposure of the spray
composition to atmospheric oxygen, making the medication
unusable. The stability of the freshly prepared spray
composition at room temperature is maintained for at least
28 days, significantly exceeding the recommended duration of
nalbuphine administration for pain relief in the majority of
clinical applications. The pharmaceutical company Microkhim
conducted a series of preclinical studies using cell cultures
and animal experiments to obtain approval from the national
regulatory agency for a phase I pharmacokinetic study on
healthy volunteers in 2021. Positive results of the phase I PK
study enabled the company to conduct a phase II clinical trial
on postoperative patients one year later.
Comparative Pharmacokinetic Study
Phase I clinical trial is the first randomized, cross over study to
compare the PK parameters and safety of nalbuphine solution
administered intravenously and intramuscularly with
intranasal administration of the nalbuphine nasal spray Apain®
in healthy volunteers. The study was carried out in the
inpatient therapeutic unit of the clinical and diagnostic center
pharmbiotest (Kyiv, Ukraine). Twenty four healthy volunteers,
15 men and 9 women, aged 18-50 years, with a body mass
index of 18 kg/m2-30 kg/m2, were enrolled in this randomized,
open label, cross over study consisting of three periods and six
sequences. In each period, the study participants received one
of the following drugs: 7.0 mg nasal spray (3.5 mg in each
nostril), nalbuphine hydrochloride solution for injection, 10
mg/ml, 1 ml intravenously and the same solution, 1 ml
intramuscularly. The nasal spray dose was selected based on
published data of a tolerable 0.1 mg/kg dose, recalculated for
the average adult body mass of 70 kg. Study participants were
closely monitored for potential respiratory depression within
24-72 hours after dosing.
A comparison of the PK profiles for Intravenous (IV),
Intramuscular (IM) and Intranasal (IN) routes of nalbuphine
administration revealed a close similarity in the absorption
phases between nasal spray and IM injection. Differences
between the mean Tmax and dose-adjusted Cmax values for the
nasal spray and IM injection were not statistically significant.
The elimination rate constants and the terminal elimination
half-life following IV, IM and IN nalbuphine administration had
similar median values. The mean absolute bioavailability of the
nasal spray Apain® equaled 65.04%. Since the nasal spray is a
hybrid medication on authorized nalbuphine solution, a
bioequivalence principle was applied to compare systemic
exposure after IM and IN administration at consecutive time
intervals corresponding to blood sampling points for PK
measurements. It was found that within 30 minutes post-dose,
the difference in systemic nalbuphine exposure between nasal
spray and IM injection was clinically insignificant (≤ 20%) and
gradually elevated reaching 37% by the fourth hour after
dosing. The study drugs were well tolerated; only non-serious
adverse reactions related to the routes of administration were
reported. PK parameters obtained for injectable routes closely
match the published data, whereas PK parameters for
intranasal nalbuphine significantly differ.
The differences could be explained by variations in the
designs, ages and conditions of the subjects enrolled in the
study, as well as the quantity and timing of blood samples
taken for PK analysis. However, the main factor contributing
to these differences was the use of different intranasal
pharmaceutical forms, i.e., specially designed spray and
injection solution. The same bodyweight dose of 0.1 mg/kg
was introduced intranasally in both studies, resulting in a
significant disparity in average bioavailability: 65% versus
41%. The similarity in the PK parameters between IM-injected
nalbuphine solution and nasal spray administration
raised the question of comparing the efficacy and safety of
these medications.
Results and Discussion
Non-Inferiority Clinical Trial
Results of the non-inferiority clinical trial establishing the
relative effectiveness and safety of the nalbuphine nasal spray
versus IM injection in postoperative patients have been
recently published. This comparative study was conducted to
assess the effectiveness and tolerance of nalbuphine nasal
spray Apain® (Microkhim, Ukraine) and nalbuphine
hydrochloride solution for injection (Hospira Inc., USA) in
patients following orthopedic and traumatological procedures.
This double blind, randomized, parallel group study was
conducted at two specialized medical centers in Kharkiv
(Ukraine) in 2021-2022. The statistical phase of the trial was
performed by CDC Pharmbiotest (Kyiv, Ukraine). It was the
first clinical trial to evaluate a specially designed nasal form of
nalbuphine.
90 postoperative male and female patients were randomly
assigned to parallel groups of 45 subjects receiving alternative
nalbuphine forms. A double dummy technique was used to
retain blinding. Patients of the test (nasal) group received the
nalbuphine nasal spray in a dose of 10.5 mg (three sprays of
3.5 mg/100 mcl) and 1 ml of placebo saline injected
intramuscularly. Subjects of the reference (IM) group received
a placebo spray of the same composition, but without the
active ingredient and 1 ml intramuscular injection of
nalbuphine hydrochloride solution (10 mg dose). Unblinded
pharmacists prepared injections before dosing under the
supervision of the study coordinator to maintain the doubleblinding
of the investigator and study participants. The main
criteria for inclusion were as follows: Age 18-70 years, body
mass index 18.5 kg/m2-35.0 kg/m2, written informed consent,
negative COVID-19 test, postoperative pain intensity
measured by a Visual Analog Scale (VAS) score ≥ 4 cm after
recovering from anesthesia. To compare the pain relieving
effectiveness of the nalbuphine medications, the VAS score
was assessed before administration and at seven consecutive
time points up to 6 hours after dosing to analyze the dynamics
of the pain relief response and to calculate the Summed Pain
Intensity Differences (SPID0-6) over the 6 hours as a
comprehensive measure of efficacy. The primary endpoint of
the study was the SPID0-6 values; the secondary endpoints
were time to onset of meaningful pain relief, duration of analgesic effect, rescue medication rate; the number of
patients in study groups who demonstrated sufficient
analgesia without remedication, the area under the curve of
pain intensity versus time for 6-hour observation and RASS
score values. The pre-specified non-inferiority margin
(representing the clinically meaningful difference in SPID0-6
means between study groups) was set at -13 mm, based on
published data regarding the validated Minimum Clinically
Important Difference (MCID) of VAS scores in patients
experiencing severe pain. Due to the potential risk of
respiratory depression following administration of the study
drug, patients were closely monitored within the first 24-72
hours after dosing.
95 people were screened and 90 patients were enrolled in the
study and completed the trial. 11 patients (5 from the nasal
and 6 from the IM group) did not complete the observation
schedule because of unsatisfactory pain relief and use of
rescue medication. Thus, the ITT safety population comprised
90 and the PP efficacy population-79 patients. Patients in the
IM and nasal groups were well matched for age, gender and
BMI and baseline pain intensity at randomization. Baseline
vas scores varied from 40 mm to 91 mm in the IM group and
from 42 mm to 95 mm in the nasal group. The homogeneity
analysis did not reveal statistically significant differences
between the study groups in demographic/anthropometric
characteristics and for the "type of intervention" indicator.
No statistically significant differences were found in the VAS
scores between postoperative patients receiving nalbuphine
nasal spray and intramuscular injections (Figure 1). However,
two trends were observed: (i) slightly lower mean VAS scores
in the NASAL group compared to the IM group, especially at
the 15-minute mark and 3-6 hours after administration and
(ii) a higher interquartile range of VAS scores in the IM group
3-6 hours post-dose, indicating greater dispersion.
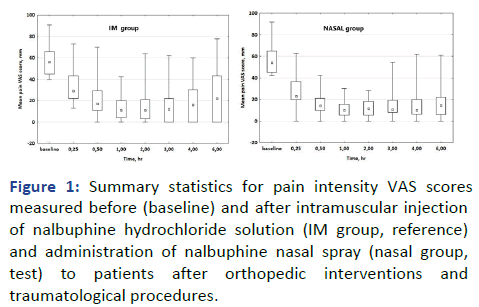
Figure 1: Summary statistics for pain intensity VAS scores
measured before (baseline) and after intramuscular injection
of nalbuphine hydrochloride solution (IM group, reference)
and administration of nalbuphine nasal spray (nasal group,
test) to patients after orthopedic interventions and
traumatological procedures.
In a box plot, the bottom and top of the box represent the 25th and 75th percentiles, the square dot inside the rectangle is the
median (50th percentile) and the bars (whiskers) represent the
minimum and maximum values. The Interquartile Range (IQR),
depicting the central portion of the data set spread, was
calculated by subtracting Q1 (25th percentile) from Q3 (75th percentile), IQR=Q3-Q1.
The primary endpoint value (the mean SPID0-6) for patients of
the nasal group, receiving nalbuphine intranasally, proved to
be slightly higher as compared to the patients of the IM group,
receiving nalbuphine intramuscularly; the difference did not
reach the statistically significant level. A comparison of
additional efficacy measures did not reveal statistically
significant differences as well, indicating a close coincidence of
the secondary endpoints' mean values between groups (Table
1). RASS scores were not analyzed statistically due to the
single occurrence of baseline and study drug-related agitation/
sedation effects and insufficient data. It has been claimed that
nalbuphine nasal spray is not inferior to intramuscular
injection since a 95% Confidence Interval (CI) for the
difference of SPID0-6 mean values included zero and the lower
limit of the 95% CI equaled -11.88 mm, which exceeded the
margin of non-inferiority.
The study participants tolerated nalbuphine well regardless of
the route of administration, but there was a greater incidence
of discomfort in the nasopharynx following nasal spray
administration (Table 2). A total of 66 adverse events were
reported in 42 patients; 23 AEs were reported in 17 patients
of the IM group and 43 AE’s in 25 patients of the NASAL
group. The severity of all AEs registered was assessed as mild or moderate; concomitant therapy for coping with AEs was
not used and AEs did not lead to study discontinuation.
Patients of the nasal group experienced more AEs related to
the nasal route of administration, however, the difference in
the number of patients experiencing AEs did not reach the
statistically significant level (p=0.091, Pearson's chi-square
test).
The results of reviewed here clinical studies examining the use
of intranasal nalbuphine are consistent with each other,
demonstrating effective pain relief and safety in both infants
and adult patients. The specially designed nasal spray
expectedly has a higher bioavailability and pain relief potency
than injection solution administered intranasally. In this
regard, the claimed non-inferiority of nalbuphine nasal spray
‘Apain’ to intramuscular nalbuphine administration has
proven the hypothesis of the previous studies that nasal
nalbuphine administration can be an adequate noninvasive
alternative to the injectable form of nalbuphine.
| Efficiency variables |
Study groups |
Total |
P value |
| IM |
Nasal |
|
The sum of pain intensity difference (SPID0-6), VAS score
|
| N (PP population)* |
39 |
40 |
79 |
|
| Mean (SD) |
228.08 (71.21) |
248.73 (73.90) |
238.53 (72.86) |
0.211# |
|
Time to onset of analgesia, h
|
| N** |
44 |
45 |
89 |
|
| Mean (SD) |
0.28 (0.09) |
0.27 (0.07) |
0.28 (0.08) |
0.703## |
|
Duration of effective analgesia, h
|
| N** |
44 |
45 |
89 |
|
| Mean (SD) |
5.55 (1.30) |
5.51 (1.41) |
5.53 (1.35) |
0.993## |
|
The number of patients who received rescue medications
|
| N (ITT population) |
45 |
45 |
90 |
|
| n (%) |
6 (13.3) |
5 (11.1) |
11 (12.2) |
0.748### |
|
The number of patients who achieved adequate pain relief
|
| N (ITT population) |
45 |
45 |
90 |
|
| n (%) |
39 (86.7) |
40 (88.9) |
79 (87.8) |
0.748### |
Note: *: Data from 11 patients were excluded due to rescue medication intake for 6-hour observation, **: Data from one patient was excluded due to the insufficient analgesic effect achieved (less than 10 mm VAS score) after study medication intake. In 10 of 11 patients, the duration of analgesia was measured as the difference between the time of rescue medication intake and the time of analgesia onset, #-Student t-test for independent samples. Datasets distribution normality was confirmed using the Shapiro-Wilk test (p>0.01), ##-Mann-Whitney U-test, ###-Pearson's chi-square test
Table 1: The average values of the treatment effectiveness metrics in patients receiving nalbuphine nasal spray and intramuscular injection.
| Number of subjects |
Study groups |
| IM n (%) |
Nasal n (%) |
| Discomfort in nasopharynx |
12 (52.2) |
16 (37.2) |
| Burning sensation |
4 (17.4) |
8 (18.6) |
| Bitter taste |
5 (21.7) |
18 (41.9) |
| Drowsiness |
1 (4.3) |
- |
| Dizziness |
1 (4.3) |
- |
| Nausea |
- |
1 (2.3) |
| In total |
23 |
43 |
Table 2: Types and incidence of adverse events experienced by patients of the IM (reference) and nasal (test) groups after receiving a single dose of study medications.
Since the absolute bioavailability of the nasal spray is 65%,
resulting in less systemic exposure compared to intramuscular
administration of injectable solutions in the same dose, the
absence of statistically significant differences in the rate,
extent and durations of pain relief effect does not match
direct exposure response relationship. Referring to PK data of
equivalent systemic nalbuphine exposure for at least thirty
minutes after the nasal spray administration and
intramuscular injection, it could be assumed that the
peripheral nociceptive system is influenced equipotently at
this time interval. Thus, the non-inferior analgesic efficacy of
the nasal spray could be attributed to heightened action on
the central nociceptive structures. Direct nose to brain
delivery by circumventing the blood-brain barrier is the initial
consideration in this regard. This assumption is supported by
the results of numerous animal studies that demonstrate the
possibility of reaching brain targets through neural
connections of the olfactory bulb and trigeminal nerve for
many pharmacological agents, including nalbuphine
nanoparticles. In the future, it may be possible to explain
these findings by comparing nalbuphine concentration profiles
in the brain tissue and/or cerebrospinal fluids after both nasal
and injectable administration, but the protocol for such
clinical study is difficult to imagine.
Conclusion
The clinical prospects of the developed nasal spray are related
to the expanding capabilities of Patient-Controlled Analgesia
(PCA), as in postoperative and acute in-hospital pain
management, as in long-term care of chronic pain in
ambulatory or home settings. Nalbuphine, the only nonscheduled
potent opioid analgesic with a wide therapeutic
window and favorable side-effect profile, has been used for
PCA since the early 80s. Nalbuphine nasal spray Apain®,
developed to be easy to use and convenient for selfadministration
and dose-adjusting by patients themselves,
has a great potential to extend PCA availability in homes
and other settings without the need for medical
personnel to administer parenteral drugs.
The nasal spray can also be used in field conditions,
emergencies, accidents and battlefields because of the
packaging in a damage-resistant plastic bottle and the longterm
stability of the medication at ambient temperatures.
Implementing nalbuphine nasal spray-based PCA in routine
medical practice will require a lot of effort.
Declaration
None.
Availability of Data and Materials
All data needed to evaluate the conclusions in the paper are
presented in the paper. Data related to this manuscript may
be requested from the authors.
Competing Interests
The authors declare that they have no competing interests.
Funding
No external funding was obtained for the study.
Authors Contributions
The study was jointly conceived, IK wrote the manuscript and
VT revised it to ensure intellectual content and exposition.
References
- Gress K, Charipova K, Jung JW, Kaye AD, Paladini A, et al. (2020) A comprehensive review of partial opioid agonists for the treatment of chronic pain. Best Pract Res Clin Anaesthesiol. 34(3):449-461.
[Crossref] [Google Scholar] [PubMed]
- Dowell D (2022) CDC clinical practice guideline for prescribing opioids for pain-United States. MMWR Recomm Rep. 71(3):1-95.
[Crossref] [Google Scholar] [PubMed]
- Fairbanks CA, Peterson CD (2023) The opioid receptor: Emergence through millennia of pharmaceutical sciences. Front Pain Res. 4:960389.
[Crossref] [Google Scholar] [PubMed]
- Eddy NB, May EL (1973) The search for a better analgesic: After 75 years of research, solutions for morphine type drug dependence are emerging. Science. 181(4098):407-414.
[Crossref] [Google Scholar] [PubMed]
- Stevens CW (2009) The evolution of vertebrate opioid receptors. Front Biosci. 14(4):1247.
[Crossref] [Google Scholar] [PubMed]
- Katritch V, Cherezov V, Stevens RC (2013) Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 53:531-56.
[Crossref] [Google Scholar] [PubMed]
- Kaufman DL, Keith DE, Anton B, Tian J, Magendzo K, et al. (1995) Characterization of the murine μ opioid receptor gene (∗). J Biol Chem. 270(26):15877-15883.
[Crossref] [Google Scholar] [PubMed]
- Yang D, Zhou Q, Labroska V, Qin S, Darbalaei S, et al. (2021) G protein-coupled receptors: Structure and function based drug discovery. Signal Transduct Tar Ther. 6(1):7.
[Crossref] [Google Scholar] [PubMed]
- Seyedabadi M, Gharghabi M, Gurevich EV, Gurevich VV (2022) Structural basis of GPCR coupling to distinct signal transducers: Implications for biased signaling. Trends Biochem Sci. 47(7):570-581.
[Crossref] [Google Scholar] [PubMed]
- Mayer P, Hollt V (2006). Pharmacogenetics of opioid receptors and addiction. Pharmacogenom J. 16:1-7.
[Crossref] [Google Scholar] [PubMed]
- Inoue A, Raimondi F, Kadji FM, Singh G, Kishi T, et al. (2019) Illuminating G-protein-coupling selectivity of GPCRs. Cell. 177(7):1933-1947.
[Crossref] [Google Scholar] [PubMed]
- Wootten D, Christopoulos A, Marti-Solano M, Babu MM, Sexton PM (2018) Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat Rev Mol Cell Biol. 19(10):638-653.
[Crossref] [Google Scholar] [PubMed]
- Urban JD, Clarke WP, Von Zastrow M, Nichols DE, Kobilka B, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 320(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- El Daibani A, Paggi JM, Kim K, Laloudakis YD, Popov P, et al. (2023) Molecular mechanism of biased signaling at the kappa opioid receptor. Nature Comm. 14(1):1338.
[Crossref] [Google Scholar] [PubMed]
- Violin JD, Lefkowitz RJ (2007) β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 28(8):416-422.
[Crossref] [Google Scholar] [PubMed]
- Smith JS, Lefkowitz RJ, Rajagopal S (2018) Biased signalling: From simple switches to allosteric microprocessors. Nat Rev Drug Discov. 17(4):243-260.
[Crossref] [Google Scholar] [PubMed]
- Wess J, Oteng AB, Rivera-Gonzalez O, Gurevich EV, Gurevich VV (2023) β-Arrestins: Structure, function, physiology and pharmacological perspectives. Pharmacol Rev. 75(5):854-884.
[Crossref] [Google Scholar] [PubMed]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, et al (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature. 537(7619):185-190.
[Crossref] [Google Scholar] [PubMed]
Citation: Kuznetsov IE, Tymko VG (2024) Nalbuphine Nasal Spray: Analgesic Efficacy and Safety. J Pharm Pharm Res. 8:011.
Copyright: © 2024 Kuznetsov IE, et al. This is an open-access article distributed under the terms of the Creative
Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the
original author and source are credited.