Keywords
Nanogels; Drug delivery systems; Drug release mechanism; Stability
Introduction
Nanogels may be defined as highly cross linked nano-sized hydrogel systems that are either co-polymerized or monomers which can be ionic or non-ionic [1,2]. The size of nanogels ranges from 20-200 nm [3]. They can escape renal clearance and prolonged serum half-life period due to their size. Nanogels are three dimensional hydrophilic networks that have the tendency to imbibe water or physiological fluid in a large amount, without changing in the internal network structure. Chemical modifications can be made to help incorporating plenty of ligands which can be used for targeted drug delivery, stimulus responsive drug release or preparation of composite materials [4]. Nanogels are known to exhibit great qualities that contribute to the drive towards it as a delivery system. They include remarkable thermodynamic stability, elevated capacity of solublilization, relatively low viscosity, and capability of undergoing vigorous sterilization techniques [5]. Nanogels may entrap drugs and biological molecules. Therefore, they can be vastly employed in protein and gene delivery. Some nanogels possess a hydrophilic nature which limits good encapsulation property of hydrophobic drugs. This issue was faced with encapsulation of anticancer drugs which are hydrophobic in nature. For this purpose, suitable structure engineering of the polymer was adopted to permit high encapsulation of them. Thereby, Nanogels provided a new mean of drug delivery for poorly soluble drugs which doesn’t only improve their solubility and stability but increasing the opportunity of their cellular uptake than the free drug [4]. Since they reflect a relatively high affinity to aqueous solutions, an outstanding stability, inertness in the systemic circulation as well as the internal fluids, and appropriateness for molecular incorporation in bulk, they are considered promising carriers for delivery and cellular uptake of proteins, peptides, and other biological compounds [6].
Properties of Nanogels
Biocompatibility and degradability: Nanogel is made up of either natural or synthetic polymers. They are highly biocompatible and biodegradable thereby avoiding its accumulation in the organs. Chitosan, ethyl cellulose, methyl cellulose and various polysaccharide-based polymers like dextran, pullulan and dextrin can be used to prepare the nanogel. Polysaccharides are mostly carbohydrate-based polymers, formed of repeating monosaccharide units linked by glycosidic bonds. These polymers are stable, non-toxic, hydrophilic and biodegradable in nature [7].
Swelling property in aqueous media: Due to the fact that Nanogels are very small, soft materials, they have the ability to swelling presence of an aqueous medium. It is considered to be the fundamental property influencing the mechanism of action followed by this drug delivery system. It depends on:
• The structure of Nanogels: This includes the Polymer chain’s chemical nature as well as cross-linking degree and in case of polyelectrolyte gels; the charge density.
• Environmental parameters which are related to the variables of the aqueous medium. For instance, in polyelectrolyte gels pH as well as ionic strength and ions’ chemical nature are influential factors. Likewise, temperature is a trigger of swelling in case of thermoresponsive gels [2].
Providing appropriate circumstances allows rapid swelling/deswelling. Regardless of the trigger, swelling takes place only when the osmotic pressure exerted by medium ions and the polymer’s network swelling pressure are imbalanced [8].
Higher drug loading capacity: Just like any other nanodelivery system, nanogels are expected to have greater loading capacity compared to conventional dosage forms. This is mainly due to the swelling property which allows the formulation to absorb large quantity of water. Thus, upon incorporation and loading the water will provide cargo space sufficient to contain salts and biomaterials [2]. Loading takes place through three methods:
Physical entrapment: it can refer to the linkage between hydrophilic chains and hydrophobic regions of the polymer or to dissolving hydrophobic molecules in hydrophilic vehicle.
Covalent attachment of bioactive molecules which leads to the formation dense drug-loaded core.
Controlled self-assembly: which is generally for polyelectrolyte-based nanogel. The high loading efficiency is attributed to interaction between oppositely charged electrolytes [2].
Other factors also contribute to the high loading capacity, such as: the composition, molecular weight, the possible interactions between the drug and the employed polymer and the different functional groups in each polymeric unit [7,9].
Permeability and particle size: What distinguishes nanodelivery systems is that a tiny manipulation in particle size, surface charge and hydrophobicity can remarkably improve permeability. In spite of the fact that nanoparticles are capable of permeation by diffusion through tissues or compromised areas of endothelium and in some cases through a particular transport system, they created a challenge crossing Blood Brain Barrier (BBB) [7]. So, in order to overcome such dilemma, nanogels were formulated in a way where they possess a diameter of 20-200 nm. It’s small enough to cross (BBB) and in the same time avoid rapid clearance mechanisms [9].
Non-immunologic response: Any agent that enters systemic circulation is rapidly eliminated by the Mononuclear Phagocyte System through opsonization and phagocytosis. Opsonization is nothing but marking foreign agents and make them visible to phagocytes. Opsonins bind on the surface of nanoparticles and facilitate the attachment of phagocytes. Few methods are adopted to help nanoparticles flee recognition and remain longer in bloodstream. All of which are based on minimizing protein binding. For example, hydrophilic polymers can act as a shield that hinders or delays binding with opsonins rendering them unnoticeable by immune system and its defenses [7,9].
Colloidal stability: When handling nanoparticle, there is always a propensity of aggregation that compromises the colloidal stability. Formulators tend to alter the surface charge to avoid the formation of aggregates in bloodstream and further complications. It can be achieved through increasing zeta potential (minimum of ± 30 mV) that results in larger repulsive forces between particles that electrostatically stabilize them. Other techniques involve the incorporation of a surface modifier like PEG that produce steric effects and hydration forces to give a stable nanosuspension [7]. If we compare polymeric micellar nanogel systems and surfactant micelles on basis of stability we will find that the former exhibits better stability lower critical micelle concentrations, decrease in dissociation rates, and longer retention of loaded drugs. They also have a high water content that assure good dispersion stability [9,10].
Advantages of Nanogels
Nanogels are considered advantageous over other drug delivery systems for a number of reasons, including:
1. High biocompatibility, which makes nanogels a very promising approach to drug delivery systems [9].
2. High biodegradability, which is crucial to avoid accumulation of nanogel material in the bodily organs, thereby leading to toxicity and adverse effects [7].
3. Nanogels are inert in the blood stream and the internal aqueous environment, meaning that they do not induce any immunological responses in the body [6].
4. Extremely small size, which induces a number of effects such as:
• Enhanced permeation capability [9].
• Avoidance of rapid renal exclusion. Escaping renal clearance leads to prolonged serum half-life [9].
• Avoidance of clearance by phagocytic cells and the uptake by reticuloendothelial system, which permits both passive and active drug targeting [9].
• Capability to cross the Blood Brain Barrier [9].
• Enhanced penetration of endothelium in pathological sites like solid tumors, inflammation tissue and infracted areas. Since Tumor tissues have a high capillary permeability, more nanoparticles permeate into the tumor tissue and accumulate there, which increases the amount of drug delivered and the selectivity of the drug delivery [6].
• Improved ability to access areas that is not accessible by hydrogels, upon intravenous administration [4].
• Safe delivery of drug carrying nanogel particles into the cytoplasm of target cells, therefore making them ideal for intracellular drug delivery.
• Rapid responsiveness to environmental changes such as pH and temperature [4].
1. Nanogels are administered via a variety of routes including oral, pulmonary, nasal, parenteral, intra-ocular and topical routes of administration.
2. Nanogels are suitable to administer both hydrophilic and hydrophobic drugs, as well as charged solutes and other diagnostic agents. This property is highly influenced by the type of functional groups present in the network of polymer chains, the crosslinking density and the type of crosslinking agent incorporated in the polymeric network [9].
3. Nanogels have a high affinity to aqueous solutions, resulting in their ability to swell or deswell, imbibing water when placed in an aqueous medium. This is the most beneficial characteristic of nanogels as it makes them ideal candidates for the uptake and delivery of proteins, peptides, bio-macromolecules as well as bulky drugs [6].
4. Drug loading in nanogels is relatively high when compared to other nanocarriers and drug delivery systems. This is due to the effect of the functional groups present in the polymeric network. By forming hydrogen bonds or other weak linkages within the polymeric network and interacting with drug or protein molecules at the interface, functional groups on the polymeric network tremendously increase the drug loading capacity of nanogels.
5. Incorporating drug into the nanogels is easy, spontaneous, and does not necessarily require any chemical reactions. This makes the process of preparing nanogels efficient, since the drug is not needed in the initial steps of the manufacturing process and can be introduced to the nanogel network in subsequent steps when the nanogel swell with water or aqueous biological fluids [4].
6. Nanogels are prepared to be capable of releasing drug in a controlled and sustained pattern at the target site, thereby enhancing the therapeutic efficacy of the drug and avoiding its adverse reactions [9].
7. Targeted drug delivery is possible in nanogels due to the presence of functional groups that conjugate with antibodies and/or drugs [9], resulting in high selectivity and preventing the accumulation of drug in non-target tissue like muscular and adipose tissue. Moreover, the chemical modification of nanogels to incorporate ligands leads to targeted drug delivery and triggered drug release [4].
8. The synthesis of nanogels is generally a stress-free process since mechanical energy is not employed and harsh conditions like sonication or homogenization are not involved [9]. Also, there is no introduction of organic solvents to the process in any of its steps. Hence the drug can be easily loaded without being exposed to any sort of vigorous conditions throughout the preparation process [4].
9. Nanogel dispersions are known to have and exceptionally large surface area which is essential for a variety of in vivo applications [4].
10. Bio-macromolecules as well as delicate compounds with low or high molecular weights can be successfully and efficiently encapsulated in the nanogels for the purpose of prolonging the activity of these molecules in the biological environment [4].
11. Nanogels can be formulated in the form of polymeric micellar nanogel systems that exhibit slower rates of dissociation, better stability over the surfactant micelles, lower critical micelle concentrations, and, most importantly, longer retention of loaded drugs [9].
Limitations of Nanogels
The only limitations to using nanogels include:
It is expensive to remove the surfactant and the solvent at the end of the preparation process although the manufacturing process itself is not very pricey.
Adverse effects may occur if any traces of polymers or surfactant remain in the body [11].
Drug Release Mechanism of the Nanogels
There are multiple mechanisms to which the release of the drug or the biomolecule is attributed to including: simple diffusion, degradation of nanogel structure, pH and temperature changes, counterion displacement or induced due to external energy source [2]. Figure 1 displays the main mechanisms of nanogel release:
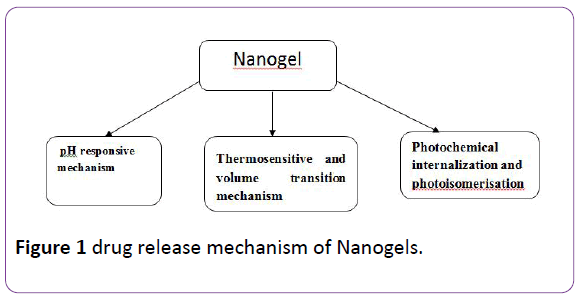
Figure 1: drug release mechanism of Nanogels.
pH responsive mechanism: As the name indicates, drug release responds to pH changes in the surrounding environment. In other words, the release of drug can take place in different physiological environments that acquire different pH values. The most release will take place in the appropriate pH which means that the release is mainly achieved in a targeted area of the body that possesses that pH. This mechanism is based on the fact that polymers employed in the synthesis of a nanogel contain pH sensitive functional groups that deionize in the polymeric network. The deprotonation results in increase in osmotic pressure, swelling and porosity of the polymer which triggers the release of the electrostatically bound molecules [2,5].
Thermosensitive and volume transition mechanism: Some nanogels are reactive to a specific temperature known as volume phase transition temperature (VPTT) which means they display a change in volume according to the temperature. If the surrounding medium is below VPTT, the polymer becomes quenched and hydrated which makes it swell and release the drug loaded. Above VPTT the opposite occurs and the nanogel shrinks abruptly and the content flows out [12]. Previously, the thermoresponsive nanogels used to rupture cellular network when they expand and increase in volume. So, some alterations were applied on thermosensitive drug-containing nanogels like changing the polymers ratio to achieve lower critical solution temperature. A good example is the biocompatible magnetic field targetibility of poly (N-isopropylacrylamide) and chitosan nanogel which is quiet employed in hyperthermic cancer treatment.
Photochemical internalization and photoisomerization: Photoisomerization refers to a process in which a bond of restricted rotation undergoes some conformational changes due to exposure to light. Double bond containing molecules are good example; they isomerize usually from a trans orientation to cis orientation upon light irradiation [13]. When photosensitizers loaded nanogel are excited, they produce two species of oxygen (singlet and reactive) which can result in oxidation in the cellular compartment walls that highly influence the release of therapeutic agents into the cytoplasm [1]. Azodextran nanogel loaded with aspirin was a subject of release studies. The observations showed that Cis-trans isomerization of azobenzene by photoregulation causes the formation of E-configuration of azo group. This results in better release profile of aspirin compared to the previous Z-configuration [1,13,14].
Classification of Nanogels
Nanogels are classified according to two basis: (Figure 2)
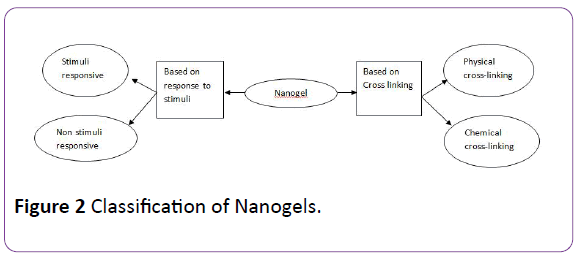
Figure 2: Classification of Nanogels.
Based on their behavior towards a specific stimuli
Non-responsive nanogels: When non-responsive nanogels come in contact with water, they absorb it, resulting in swelling of the nanogel.
Stimuli-responsive nanogels: Environmental conditions, such as temperature, pH, magnetic field, and ionic strength, control whether swelling will occur or not and the extent of swelling or deswelling of the nanogels. Any changes in any of these environmental factors, which act as stimuli, will lead to alteration in the behavior of the nanogels as a response, hence the term stimuli-responsive nanogels.
Nanogels that are responsive to more than one environmental stimulus are termed as multi-responsive nanogels [9].
Based on the type of linkages present in the network chains of polymeric gel structure
Physically cross-linked nanogels: Physically cross-linked nanogels, which are also called pseudo gels, depend greatly on the characteristics of the polymer used in their production including polymer composition, temperature, concentration of the polymer, type of cross-linking agent, and the ionic strength of the medium. Weak linkages like [1] van der Waals forces, [2] hydrogen bonding or [3] hydrophobic, electrostatic interactions are the forces that form this type of nanogels. Physical crosslinked nanogels can be produced within a short time via a number of simple methods. These methods involve a variety of processes such as association of amphiphilic blocks, self-assembly, aggregation of polymeric chains as well as complexation of oppositely charged polymeric chains.
Liposome Modified Nanogels - Liposome modified nanogels are physically cross-linked, stimuli-responsive nanogels, which are being studied as transdermal drug delivery devices, due to their unique properties. These nanogels involve the incorporation of poly [N-isopropyl-acrylamide] co-polymeric groups into the liposomes, resulting in a high degree of responsiveness to both pH and temperature. In addition, Succinylated poly[glycidol]s are infused to the liposomes, under pH of less than 5.5, in order to create nanogels that effectively and efficiently deliver Calcein to the cytoplasm of target cells. [15]
Micellar Nanogels - Micellar nanogels are produced by supramolecular self-assembly of both hydrophilic and hydrophobic blocks or by graft copolymers in an aqueous solution. Micellar nanogels consist of a hydrophilic shell (corona), made of polymer blocks, surrounding a hydrophobic core, and stabilizing the whole micelle. The purpose of this conformation is to provide sufficient space to contain drugs or biological macromolecules just by physically entrapping these particles inside the borders of the shell, thereby acting as a drug delivery system. As the micelle enters the body, the hydrophilic shell interacts with the aqueous media by forming hydrogen bonds in order to protect the hydrophobic core that is carrying the drug to its target cells. This process protects the drug molecules from being hydrolyzed or degraded by enzymes.
Hybrid Nanogels - When particles of a nanogel are dispersed in organic or inorganic medium, it is known as a Hybrid nanogel. Self-assembly and aggregation of amphiphilic polymers, such as pullulan-PNIPAM, hydrophobized polysaccharides, and hydrophobized Pullulan, were the processes used for the formation of nanogels in aqueous medium (Akiyoshi et al., 1999; Akiyoshi et al., 2000). Specifically, cholesterol-bearing pullulan (CHP) nanogels were investigated. These are stable monodispersed nanogels formed by the self-aggregation of CHP molecules (formed of pullulan backbone and cholesterol branches) with hydrophobic groups providing physical crosslinking points (Figure 3) CHP nanogels were found to have the unique abilities to not only complex with molecules like DNA, proteins and various drugs but also to coat solid surfaces like liposomes, particles and even cells (Nishikawa et al., 1996; Kuroda et al., 2002). Hybrid nanogels have significance, particularly, as drug delivery systems for insulin and anticancer drugs [9].
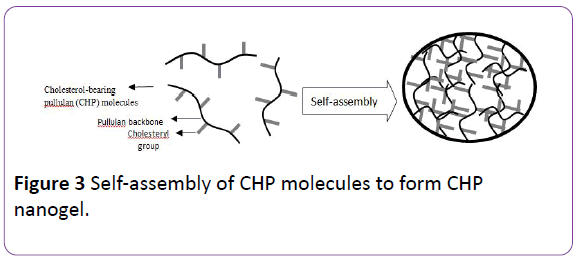
Figure 3: Self-assembly of CHP molecules to form CHP nanogel.
Chemically cross-linked nanogels: Where physically cross-linked nanogels are linked by weak forces, chemically cross-linked nanogels are formed by networks of strong covalent bonds and other permanent chemical linkages. The strength of the linkage is highly dependent on the type of functional groups present in the molecules of the nanogel network.
In order to synthesize this type of nanogels, polymeric chains are cross-linked at specific points, called the cross-linking points, which are determined by the multifunctional cross-linking agent present. Using different polymers and different chemical linking strategies leads to the production of nanogels with a variety of properties for a number of applications. In addition, the physiochemical properties of the nanogel can be modified depending on the type of cross-linking agent used to produce the polymer and the position of cross-linking points. Hydrophilic polymers or amphiphilic copolymers, produced by polymerization of vinyl monomers, are usually used to produce chemically cross-linked nanogels.
For example, a nanogel ranging in size from 20 to 200 nm, in which polymeric chains containing pendant thiol groups were crosslinked by an environment friendly chemical method, was produced (Aliyer et al., 2005) [9].
Synthesis of Nanogels
Photolithographic techniques
Photolithographic techniques, photochemical reaction for activation and subsequent reaction have been explored in strive of producing 3D hydrogel particles and nanogels for drug delivery. In this method, stamps or replica molds are treated to give the surface specific properties that allow the molded gels to release the incorporated agents [16]. Microfabrication of such gels follow the general strategy where poly (dimethylsiloxane) (PDMS) stamps are utilized to mold, release, and stack gels into 3 dimensional structures. Surface modification enhances the release or adhesion of molded gels to a substrate. The most known techniques to modify PDMS stamps are usually achieved by hexa (ethylene glycol)-terminated self-assembled monolayers (SAMs), or by adsorbed monolayers of bovine serum albumin (BSA) [17].
Modified pullulan technique
The example that can be given for this category is selfassembled hydrophobized pullulan nanogel. The pullulans are modified in to stages; initially methacrylates are used, then with hydrophobic 1-hexadecanethiol. The end product is an amphiphilic material that upon addition of water starts to assemble itself by hydrophobic interaction among alkyl chains [18].
Another example is Cholesterol based pullulan nanogel. Here, pullulan was substituted with 1.4 cholesterol and the nanogel is fabricated by simply reacting cholesterol isocynate in dimethyl sulfoxide and pyridine. This mixture was freeze dried and in aqueous phase it formed nanogel which further formed a complex with W-9 peptide, a TNF-alpha and RANKL antagonist for delivery of osteological disorder [19].
Cholesteryl pullulan (CHP) bearing methacrylol was formulated by the reaction of CHP with glycidyl methacrylate. The degree of substitution was 6.2 per 100 glucose unit (CHPMA6). CHPMA6 formed nanogel in water self-assembly [20].
Emulsion polymerization technique
l-proline functionalized PMMA [poly (methyl methacrylate)] nanogel with a range of catalyst functionalization (0.5-15 wt%) and cross linking densities (0-50 wt%) were prepared by the emulsion polymerization technique [21]. In emulsion polymerization technique monomer droplets are formed by mechanical stirring [22].
Reverse microemulsion polymerization technique
Lithium loaded Polyacrylic acid (PAA) nanogels were formulated by reverse microemulsion polymerization technique. 3.43 g span 80 & 2.62 g span 80 were added to 100 ml hexane that is oil phase and kept for stirring using magnetic stirrer. Aqueous phase was prepared by adding 1.5 ml of 10% (w/w) LiOH in water to 500 μl acrylic acid. Add 214 μl of 5% (w/v) N, N’-Methylenebisacrylamide (MBA) suspension, 500 μl of 2% (w/v) potassium persulfate and 40 μl of 20% (w/v) N, N, N’, N’- Tetramethylene-diamine (TEMED) to aqueous phase [23].
Microemulsion was formed by adding aqueous phase drop wise into oil phase. Emulsion was transferred to 60°C water bath and stirred at 400 rpm using magnetic stirrer, kept overnight at room temperature. Supernatant were decanted and pellets were collected. Microemulsion is thermodynamically stable [23].
Inverse miniemulsion polymerization technique
Fluorescent dye Rhodamine B or Fluorescein labeled nanogels were prepared by activators generated electron transfer atom transfer radical polymerization (AGET ATRP) of oligo (ethylene oxide) monomethyl ether methacrylate (OEO300MA) by inverse mini-emulsion polymerization of water/cyclohexane at ambient temperature. Hydroxyl containing ATRP initiator was used to control polymerization to produce functional HO-POEO300MA nanogels. Cell adhesive nanogels were synthesized using ACRLPEO- GRGDS as a co-monomer during the polymerization [3]. In O/W mini-emulsion technique monomer droplets are formed by applying high shear stress by ultrasonication or high pressure homogenizer. Miniemulsion is kinetically stable [22].
Free radical crosslinking polymerization technique
Photocrosslinked biodegradable photoluminescent polymers (PBPLPs) nanogel was prepared by free radical crosslinking of a vinyl-containing florescent prepolymer for drug delivery and cell imaging. Development of PBPLPs nanogel shows a new era to develop nanobiomaterials in theranostic nanomedicine for drug delivery and cell imaging [24].
Applications of Nanogels
Local anesthetics (LA): Local anesthetics are one of the classes of drugs that induce analgesia and eliminate pain. The analgesic effect of local anesthetics is due to the blockage of the nerve impulses in nerve cell membrane by shutting the voltage gated Na+ channels. The manner and the intensity of nerve stimulation as well as its resting membrane potential will determine the degree of numbness induced by a specific concentration of a local anesthetic. Local anesthetics are clinically classified into two classes, depending on their chemistry: amino esters and amino amide. Over dosage of local anesthetics leads to their high toxicity, which has sparked the interest in formulating controlled release drug delivery systems of them. Incorporating local anesthetics into drug delivery systems like nanogels can improve their regional administration. A delivery system of procaine hydrochloride, which is an amino ester local anesthetic, loaded into methacrylic acid ethyl acrylate nanogel via hydrophobic and hydrogen bonds exhibited a high release rate at high pH. The mechanism of release is based on the deprotonation of the acid on the nanogel which leads to an increase in the osmotic pressure and the swelling of the whole system, which increases the porosity, thus promoting the release of the procaine hydrochloride [5].
Cancer treatment: Biodegradable nanogel prepared by cross linking of polyethyleneimine and PEG/pluronic used for 5’- triphosphorylated ribavirin reduced toxicity [25]. Doxorubicine loaded self-organizing nanogel formulated by acetylated chondroitin sulphate used for cancer treatment [26]. pHresponsive doxorubicine uptake accelerated nanogel containing glycol chitosan, which was grafted with 3- diethylaminopropyl groups [27]. Self-quenching polysaccharide based pullulan/ folate-pheophorbide used in minimal toxicity of pheophorbide [28]. Cross linked branched network of polyethyleneimine and PEG [Polyplex nanogel] used for elevated activity and reduced toxicity of fludarabine [29]. Self-assembled nanogel composed of heparin pluronic used to deliver RNaseA enzyme to internalize in cell [30]. Cholesterol bearing pullulan sustained release nanogels used in recombinant murine onterlikine-12 sustained tumor immunotherapy [31]. Reducible heparin with disulfide linkage nanogel used in internalization of heparin for apoptotic death of melanoma cells [32]. Specific targeting nanogel of doxorubicin loaded acetylated hyaluronic acid used in cancer treatment [33]. pH and temperature responsive cadmium (II) ions quantum dots, made of Hydroxypropylcellulose – poly (acrylic acid) used in cell imaging [34]. In-situ Poly (Nisopropylacrylamide- co-acrylamide) gelatinized thermosensitive nanogel used to deliver 5-fluorouracil [35]. Cholesterol bearing pullulan with modified amino group, quantum dot hybrid nanogel used for bioimaging [36]. Generally, nanoparticles possess an average diameter of nearly 100 nm, neutrality and surface hydrophilicity which results in a prolonged blood circulation and an increased level of tumor delivery [37].
Autoimmune disease: The treatment of autoimmune disorders is based on the ability of the drug delivery system to selectively disable the immune cells that mediate the autoimmunity response. The incorporation of immunosuppressant drugs into nanogel delivery systems have been extensively studied for this purpose since nanogels can improve the immunosuppression effect by targeting the antigen presenting cells that contribute to disease and enabling systemic accumulations of the loaded drug. A nanogel system of mycophenolic acid compexed with non-methylated β- cyclodextrin was formulated by loading of liposomes with a diacrylate terminated copolymer of poly (lactic acid-co-ethyleneglycol) and tested for the treatment of systemic lupus erythematosus, an autoimmune disease. The cross linking between acrylated monomers and the gelation of the particles into a stable mix was achieved by exposing the nanogel system to ultraviolet radiation.
Neurodegenerative disease: Currently, neurodegenerative disorders like Alzheimer’s & Parkinson’s disease have no known cure, therefore, when oligonucleotides showed a potential to be used as a diagnostic or therapeutic tool for these diseases, they became the focus of many studies. So far, the application of oligonucleotides in the treatment of neurodegenerative disease is significantly hindered by their instability against metabolism, their inability to penetrate the blood brain barrier, and their rapid clearance by renal excretion. To enhance the performance of oligonucleotides, they were incorporated into nanogel delivery systems. The novel properties of nanogels allow oligonucleotides to cross the blood brain barrier, thereby aiding their delivery into the central nervous system. A nanogel of oligonucleotide, which was formulated by crosslinking poly (ethylene glycol) and polyethylenimin, was found to have the ability to form a stable aqueous dispersion of polyelectrolyte complex by encapsulating negatively charged particles of the drug. Modifying the surface with insulin or transferrin, results in enhanced transport efficacy [38].
Anti-inflammatory: Nanogels have found an application dermatology and cosmetology as topical delivery systems of non-steroidal anti-inflammatory drugs (NSAIDs) and for the treatment of allergic contact dermatitis and psoriatic plaque. Nanogels are ideal for this application since they can overcome the major limitation of topical delivery systems, which is the relatively short contact time between active drugs and the application site. This is done by retaining water into the gel matrix and forming a uniform a dispersion of the nanogel. The simultaneous topical delivery of two anti-inflammatory drugs, Spantid II and ketoprofen was successfully achieved through a nanogel of poly-(lactide-co-glycolic acid) and chitosan. Oleic acid was used for surface modification. A variety of inflammatory disorders can be treated using this nanogel system as it can effectively permeate to deep layers of the skin [39].
Vaccine delivery: Vaccination is based on the induction of an immune response that antigen-specific. In order to enhance the potency and the performance of vaccines, polymeric nanogels are being utilized as novel, alternative means of vaccine delivery. The advantage of nanogels over conventional vaccines lies in the ability of the nanogel network to protect vaccine antigens from enzymatic degradation. Target specificity of the vaccine delivery can be significantly enhanced by using surface modified nanogels with attached antibodies and other ligands [40].
Transdermal drug delivery: Transdermal route of administration has advantages over other routes in that it bypasses first pass effect, improves the efficiency of drugs, provides steady state drug concentration in plasma and increases patient compliance. A variety of approaches were considered to enhance the penetration of drug into site of action. A promising approach is the use of nanogels for topical delivery of active pharmaceutical ingredients to the stratum corneum. As the oral administration of aceclofenac causes a number of side effects like ulcers and gastric bleeding, transdermal delivery of the drug, was studied as an alternative, and showed better stability and permeability. Through the emulsion solvent diffusion method, a dispersion of aceclofenac was formed and incorporated into a gel matrix to formulate a nanogel for the transdermal delivery of the drug [41].
Bone regeneration: For the successful regeneration of bones, biodegradable cell scaffolds should release lithium as well as other medicament slowly and locally. Bone growth can be increased by lithium, hence, lithium nanogels, synthesized by micro-emulsion polymerization of polyacrylic acid and incorporated into the biodegradable polyhydroxybutyrate matrix, are formulated for the controlled release of lithium into bone tissue [23].
Antibacterial and anti-microbial activity: Infections are becoming increasingly difficult to cure due to resistance to conventional delivery systems of antibiotics. In order to treat a microbial infection, a quick and localized action is required, which is possible in nanogel delivery systems. Dextran crosslinked polyacrylamide nanogels (polysaccharide based nanogels) loaded with zinc nitrate (zinc ions) as antibacterial agent were prepared by mini-emulsion method. The crosslinking agent used was methacrylated hyaluronic acid. The purpose of this nanogel was to target the methicillin-resistant strains of staphylococcus aureus [42].
Diabetics: As diabetes becomes more and more prevalent in the world’s population, revolutionized approaches are being considered for its treatment. An injectable nanogel network that is sensitive to changes of glucose levels in the blood and releases specific amounts of insulin accordingly has been formulated, containing a network of oppositely charged nanoparticles. These nanoparticles attract each other, forming a gel matrix that remains intact and responds to changes in pH. By utilizing dextran, the nanogel network will carry insulin and other enzymes necessary for the conversion of glucose into gluconic acid. Under conditions of hyperglycemia, glucose molecules, being easily diffusible through the nanogel, pass the gel network and trigger the conversion process of glucose into gluconic acid, thereby decreasing the pH of the medium. This will, in turn, stimulate the release of insulin. Even though this approach is very promising for the treatment of diabetes, it is still new and needs some work to be done before this nanogel is suitable for human trials [43].
Ophthalmology: Dexamethasone containing eye drop was prepared by solvent evaporation or emulsification method using 2-hydroxypropyl-γ-clclodextrin (HP γ CD) medium containing γ- CD nanogel for sustain release. pH-sensitive polyvinylpyrrolidone-poly [acrylic acid] (PVP/PAAc) nanogels, formulated by γ radiation-induced polymerization of acrylic acid (AAc) in an aqueous solution of polyvinylpyrrolidone (PVP) acting as a template, were used to encapsulate pilocarpine, thus enhancing the bioavailability as well as the stability of pilocarpine and maintaining an adequate concentration of the drug at the site of action for prolonged period of time [44,45].
Conclusion
As a new and improved approach to diagnosis and treatment of a wide range of diseases, nanogels have been proved to bring about huge advancements in this field. Nanogels have versatile properties that make them capable of efficient delivery of biologically active molecules, particularly biopharmaceuticals. This has given rise to a number of therapeutic applications; nanogels are used in the controlled delivery of active drug compounds. They can also be used as a carrier, or chaperone, to treat diabetes, cancer, neurodegenerative disease, etc. Unique properties of nanogels, like their tailoring characteristics and easy encapsulation of therapeutics, have promoted these applications of nanogels. They can also minimize the side effects of drugs and lower their therapeutic dose, resulting in improved efficacy of therapeutic agents and increased benefit to the patient.
References
- DhawalDorwal(2012)NanogelsAs Novel And Versatile Pharmaceuticals International Journal of Pharmacy and Pharmaceutical Sciences 4: 67-74.
- Kabanov AV1, Vinogradov SV (2009) Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. AngewChemInt Ed Engl 48: 5418-5429.
- Bencherif SA, Siegwart DJ, Srinivasan A, Horkay F, Hollinger JO, et al.(2009) Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials 30: 5270–5278.
- Soni G, Yadav KS (2016)Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm J 24: 133-139.
- Tan JP, Tan MB, Tam MK (2010) Application of nanogel systems in the administration of local anesthetics.LocalRegAnesth 3: 93-100.
- Rigogliusoa S, Sabatinob MA, Adamoa G, Grimaldib N, Dispenzab C, et al. (2012)Nanogels: Nanocarriers For Drug Delivery Application. Chemical Engineering Transactions 27:247-252.
- Gonçalves C, Pereira P, Gama M (2010)Self-Assembled Hydrogel Nanoparticles for Drug Delivery Applications. Materials 3:1420-1460.
- Kazakov S, Levon K (2006) Liposome-Nanogel Structures for Future Pharmaceutical Applications. Curr Pharm Des 12:4713-4728.
- Sultana F, Manirujjaman, Md Imran-Ul-Haque, Arafat M, Sharmin S (2013)An Overview of Nanogel Drug Delivery System. JAppl Pharm Sci3: 95-105.
- Vinogradov SV (2010)Nanogelsin the race for drug delivery. Nanomedicine5:165–168.
- Singh N, Nisha, Gill V, Gill P(2013) Nanogel Based Artificial Chaperone Technology: an Overview.American Journal of Advanced Drug Delivery. American jadva drug del1:271-276.
- Lu X, Sun M, Barron AE (2011)Non-ionic, thermo-responsive DEA/DMA nanogels: Synthesis, characterization, and use for DNA separations by microchip electrophoresis. J Colloid Interface Sci357 : 345–353.
- Fomina N, Sankaranarayanan J, Almutairi A (2012)Photochemical mechanisms of light-triggered release from nanocarriers. Adv Drug Deliv Rev 64: 1005–1020.
- Patnaik S, Sharma AK, GargBS, Gandhi RP, Gupta KC (2007)Photoregulation of drug release in azo-dextran nanogels. Int J pharm 342:184-193.
- Labhasetwar V, Leslie-Pelecky DL (2007)Biomedical applications of nanotechnology. John Wiley & Sons.
- Oh JK, Drumright R,Siegwart DJ, Matyjaszewski K (2008)The development of microgels/nanogels for drug delivery applications. ProgPolymSci33:448–477.
- Tang MD, Golden AP, Tien J (2003)Molding of Three-Dimensional Microstructures of Gels. J Am ChemSoc 125:12988-12989.
- Ferreira SA, Coutinho PJG, Gama FM (2011) Synthesis and Characterization of Self-Assembled Nanogels Made of Pullulan. Materials 4:601-620.
- Alles N, Soysa NS, Hussain MA, Tomomatsu N, Saito H, et al.(2009) Polysaccharide nanogel delivery of a TNF-α and RANKL antagonist peptide allows systemic prevention of bone loss. Euro J Pharm Sci37:83-88.
- Akiyoshi K (2007)Nanogel-based Materials For Drug Delivery System. European Cells and Materials14:36.
- Lu A, Moatsou D,Longbottom DA, O’Reilly RK (2013) Tuning the catalytic activity of L-proline functionalized hydrophobic nanogel particles in water. ChemSci 4: 965-969.
- Sanson N,Rieger J (2010) Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. PolymChem1:965–977.
- LarssonM, Bergstrand, A, Mesiah, L, Vooren CV, Larsson SA(2014)Nanocomposites of polyacrylic acid nanogels and biodegradable polyhydroxybutyrate for bone regeneration and drug delivery. J Nanomaterials2014:1-9.
- D Manry, D Gyawali, J Yang (2011) Size optimization of biodegradable fluorescent nanogels for cell imaging. . High School Res 2: 1.
- Kohli E, Han HY, Zeman AD, Vinogradov SV (2007) Formulation of biodegradable nanogel carriers with 5’-triphosphates of nucleoside analogs that display a reduced cytotoxicity and enhanced drug activity. J Controlled Release121:19-27.
- Park W, Park SJ, Na K(2010) Potential of self-organizing nanogel with acetylated chondriotin sulfate as an-anti-cancer drug carrier. Colloids Surf B 79:501-508.
- Singka GSL, Samah NA, Zulfakar MH, Yurdasipe A, Heard CM (2010) Enhanced topical delivery and anti-inflammatory activity of methotrexate from an activated nanogel. Euro J Pharm Biopharm40:234-253.
- Bae B, Na K (2010) Self-quenching polysaccharide based nanogels of pullulan/folate-photosensitizer conjugates for photodynamic therapy. Biomaterials 31:6325-6335.
- Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV (2005)Polyplexnanogel formulation for drug delivery of cytotoxic nucleoside analogs. J Controlled Release 107: 143-157.
- Choi JH, Jang JY, Joung YK, Kwon MH, Park KD (2010) Intracellular delivery and anti-cancer effect of assembled heparin-pluronicnanogel with RNaseJ Control Release 2:32-45.
- Shimizu T, Kishida T, Hasegawa U, Ueda Y, Imanishi J, et al.(2008) Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. BiochemBiophys Res Commun 367: 330-335.
- BaeKH, Mok H, Park TG (2008) Synthesis, characterization and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials 29:3376-3383.
- Park W, Kim KS, Bae B, Kim Y, Na K (2010) Cancer cell specific targeting of nanogels from acetylated hyaluronic acid with low molecular weight. Euro J Pharm Sci 40:367-375.
- Wu W, Aiello M, Zhou T, Bernila A, Banerjee P, et al. (2010) In situ immobilization of quantum dots in polysaccharide based nanogel for integreation of optical pH sensing, tumor cell sensing and drug delivery. Biomaterials 31:3023-3031.
- Wang Q, Xu H, Yang X, Yang Y (2008) Drug release behavior from in situ gelatinized thermosensitivenanogel aqueous dispersions. Int J Pharm 361:189-193.
- Hasegawa U, Nomura ICM, Kaul SC, Hirano T, Akiyoshi K (2005]Nanogel quantum dots hybrid nanoparticles for live cell imaging. BiochemBiophys Res Commun 331:917-921.
- Look M1, Stern E, Wang QA, DiPlacido LD, Kashgarian M, et al.(2013)]Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. J Clin Invest123:1741–1749.
- Vinogradov SV1, Batrakova EV, Kabanov AV (2004)Nanogels for Oligonucleotide Delivery to the Brain.BioconjugChem15:50–60.
- Shah PP, Desai PR, Patel AR, Singh M (2012) Skin permeating nanogel for the cutaneous co-delivery of two anti-inflammatory drugs.Biomaterials 33: 1607–1617.
- Ferreira SA1, Gama FM, Vilanova M (2013)Polymeric nanogels as vaccine delivery systems. Nanomedicine 9:159–173.
- Phatak AA, Praveen DC (2012) Development and Evaluation of Nanogel as a Carrier for Transdermal Delivery of Aceclofenac. Asian J Pharm Tech 2:125-132.
- Malzahn K,Jamieson WD, Droge M,Mailander V , Jenkins ATA, et al.(2014) Advanced dextran based nanogels for fighting Staphylococcus aureus infections by sustained zinc release. J Mater Chem B 2: 2175–2183.
- Paddock C (2013) Nanogel to Manage Type 1 Diabetes. Medical News Today.
- Moya-Ortega MD, Alves TF, Alvarez-Lorenzo C, Concheiro A, Stefánsson E, et al.(2013)Dexamethasone eye drops containing γ-Cyclodextrin based nanogel. International journal of Pharmaceutics 441: 507-515.
- Abd El-Rehim HA1, Swilem AE, Klingner A, Hegazy el-SA, Hamed AA (2013)Developing the potential ophthalmic applications of pilocarpine entrapped into polyvinylpyrrolidone-poly[acrylic acid] nanogel dispersions prepared by γ radiation. Biomacromolecules14:688-698.