- (2015) Volume 16, Issue 2
Anthony Sabo1, Naeem Goussous1, Neeraj Sardana2, Shirali Patel1 and Steven C Cunningham1*
Departments of 1Surgery and 2Medicine, Saint Agnes Hospital, Baltimore, USA
Received January 5th, 2015 – Accepted February 20th, 2015
The objective of this review is to summarize the current state of the art of the management of necrotizing pancreatitis, and to clarify some confusing points regarding the terminology and diagnosis of necrotizing pancreatitis, as these points are essential for management decisions and communication between providers and within the literature. Acute pancreatitis varies widely in its clinical presentation. Despite the publication of the Atlanta guidelines, misuse of pancreatitis terminology continues in the literature and in clinical practice, especially regarding the local complications associated with severe acute pancreatitis. Necrotizing pancreatitis is a manifestation of severe acute pancreatitis associated with significant morbidity and mortality. Diagnosis is aided by pancreas-protocol computed tomography or magnetic resonance imaging, ideally 72 h after onset of symptoms to achieve the most accurate characterization of pancreatic necrosis. The extent of necrosis correlates well with the incidence of infected necrosis, organ failure, need for debridement, and morbidity and mortality. Having established the diagnosis of pancreatic necrosis, goals of appropriately aggressive resuscitation should be established and adhered to in a multidisciplinary approach, ideally at a high-volume pancreatic center. The role of antibiotics is determined by the presence of infected necrosis. Early enteral feeds improve outcomes compared with parenteral nutrition. Pancreatic necrosis is associated with a multitude of complications which can lead to long-term morbidity or mortality. Interventional therapy should be guided by available resources and the principle of a minimally invasive approach. When open debridement is necessary, it should be delayed at least 3-6 weeks to allow demarcation of necrotic from viable tissue
Debridement; Endoscopy; Octreotide; Pancreatitis, Acute Necrotizing; Stents
Acute pancreatitis results in nearly 250,000 annual admissions at a cost of approximately $2.2 billion [1, 2]. Necrosis complicates as many as 20% to 30% of all cases of acute pancreatitis, and these cases of necrotizing pancreatitis (NP) are associated with a markedly increased morbidity and mortality (as high as >60%) [3-7]. Mortality seen early in the clinical course is often a result of end organ failure, most commonly renal and pulmonary failure, whereas late mortality is often the result of an infectious process.
Although the risk factors for NP and uncomplicated pancreatitis are largely similar, there are some notable exceptions. The etiology in both types is vastly likely to be either gallstones or alcohol [1]. Other well-known etiologies include medications, hypertriglyceridemia, instrumentation of the pancreatic duct or ampulla, obstructing neoplasms or stones, pancreaticobiliary malformations, genetic defects, and autoimmune disorders [1]. In addition, NP has been associated temporal if not causally, with extended exposure to transcutaneous nerve stimulation devices [8]. Indeed, an increasing proportion of cases are labeled as idiopathic [9-11]. One very common etiology of acute pancreatitis, however, alcohol, has been identified as a particularly strong risk factor for developing necrosis [12] but there are conflicting data [13].
This review will emphasize current literature on NP and elucidate terminology, imaging considerations, complications, assessment of severity, and management.
The original Atlanta classification [14], responded to what was at the time a confusing medley of terms, used with a marked inconsistency in the literature [15]. More recently, the 2012 revised Atlanta classification [3, 16] for acute pancreatitis addressed several lingering deficiencies and further developed consistent terminology for acute pancreatitis and its sequelae (Table 1). The term mild acute pancreatitis (MAP) is now defined as pancreatitis without organ failure (defined below, such as renal or pulmonary failure), or complications (such as necrosis or pseudocysts), as discussed below. Moderately severe acute pancreatitis (MSAP) is defined by organ failure lasting <48 hours, or by local complications. And the term severe acute pancreatitis (SAP) is reserved for cases in which organ failure lasts >48 h [3, 16].
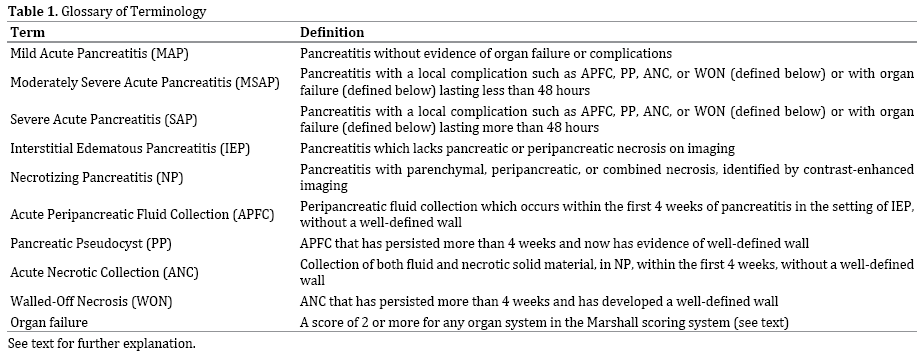
According to the current classification of acute pancreatitis, interstitial edematous pancreatitis (IEP) is defined by the lack of pancreatic or peripancreatic necrosis on imaging, and is distinguished from NP, which is subdivided into three categories: parenchymal necrosis, peripancreatic necrosis, or combined necrosis, all three of which may be infected or sterile [3, 16]. The disease process is further separated into an early phase and a late phase, with definition of local complications based on characteristics of collections of fluid and necrosis.
In the setting acute pancreatitis, typically IEP, a peripancreatic fluid collection occurring within the first 4 weeks is termed an acute peripancreatic fluid collection (APFC) and is characterized by the lack of both a welldefined wall and pancreatic or peripancreatic necrosis on imaging [3, 16]. When a APFC persists beyond 4 weeks, a well-defined wall will develop and the term pancreatic pseudocyst (PP) is applied [3, 16, 17]. Similarly, in the setting of NP, a collection of not only fluid but also necrosis involving the pancreatic parenchyma or the peripancreatic tissues is termed an acute necrotic collection (ANC) when seen within the first four weeks of the disease. Like APFCs, ANCs lack a well-defined wall. When an ANC persists beyond four weeks and becomes encapsulated, the term walled-off necrosis (WON) is used [3, 16] (Figure). Concisely, a APFC contains no necrotic material, whereas ANC contains fluid and necrosis; when these two entities persist beyond 4 weeks, they become PP and WON, respectively.
Erstwhile terms, such as pancreatic abscess, pancreatic sequestration, necroma, and organized pancreatic necrosis, have fallen out of favor, and their use should be discouraged to avoid confusion.
Because the revision of the Atlanta classification relies so heavily on morphologic criteria for defining the various sequelae of acute pancreatitis computed tomography (CT), typically pancreas-protocol CT (PPCT) with both an early arterial phase and a portovenous phase, ideally using bolus-tracking to avoid mis-timings related to cardiac output [18, 19], is essential for identifying the sequelae of acute pancreatitis. Although CT is not required on presentation in all cases of acute pancreatitis, unless necessary to rule out other pathology, it is often used on presentation due to its wide availability and high degree of accuracy. The ideal time for assessing the sequelae of acute pancreatitis with PPCT is after 72 hours from onset of symptoms since edematous or transiently ischemic parenchyma masquerading as necrosis may resolve on subsequent imaging, and local complications not initially present can subsequently develop without clear clinical correlates [20-25]. Repeat CT imaging is also indicated when the clinical picture significantly changes, as may happen with fevers, decrease in hematocrit, or sepsis. CT is of course an indispensible adjunct to guide needle and catheter placement and is used to assess success of treatment in patients having undergone percutaneous, endoscopic, or operative interventions.
Interstitial Edematous Pancreatitis
In patients with IEP, PPCT demonstrates localized or diffuse enlargement of the pancreas, with normal homogeneous enhancement or slightly heterogeneous enhancement of the pancreatic parenchyma related to edema. The peripancreatic and retroperitoneal tissue may appear normal, usually in early mild disease, or may show mild inflammatory changes in the peripancreatic soft tissue that appear as mild fat stranding with varying amounts of peripancreatic fluid. In cases of heterogeneous or questionable enhancement seen at PPCT does early (<72 hours) in the course of the disease, the presence or absence of pancreatic necrosis may be initially indeterminate. As described above, PPCT performed 3–7 days later permits more definitive characterization [20, 25, 26].
Necrotizing Pancreatitis
When any region of the pancreas demonstrates an area of attenuation <30 Hounsfield units during the early arterial phase, pancreatic necrosis may be diagnosed [20-27]. The percentage of the gland that is necrotic on PPCT has been shown in numerous studies to predict the development of infected necrosis [28-30], need for necrosectomy [31, 32], organ failure [30], and overall morbidity and mortality [7, 30, 33] (Table 2).
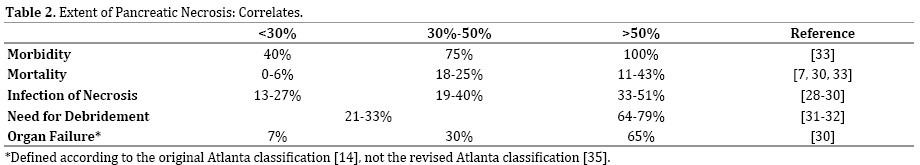
Pancreatic parenchymal necrosis alone, however, is seen in only 5% of patients and peripancreatic necrosis alone is seen in only 20% of patients and can be difficult to confirm [26, 34, 35]. Its presence is diagnosed when heterogeneous areas of nonenhancement are visualized to contain nonliquid components, commonly located in the retroperitoneum and lesser sac. The most common type of NP is combined pancreatic parenchymal necrosis with peripancreatic necrosis [26, 34, 35]. All three types can be sterile or infected, although infection may be present with or without the telltale gas sometimes seen on CT [20, 25, 26, 35].
Pancreatic and Peripancreatic Collections
APFCs typically arise in patients with interstitial edematous pancreatitis during the first 4 weeks. Radiographically, they conform to the anatomic boundaries of the retroperitoneum, particularly the anterior pararenal fascia, and are usually seen immediately next to the pancreas but have no clearly defined wall. After the first 4 weeks from onset of acute IEP, an APFC may persist as a PP. On PPCT, PPs are well-circumscribed, usually round or oval peripancreatic fluid collections of homogeneously low attenuation that are encapsulated by a well-defined mildly enhancing wall consisting of fibrous or granulation tissue [20, 26].
ANCs also develop in the first 4 weeks but they arise in the setting of NP and contain both fluid and necrotic material of various amounts (some of which may be loculated), which produce a heterogeneous appearance on PPCT and magnetic resonance imaging (MRI) not seen with APFC or with PPs [20, 26]. As a ANC matures beyond 4 weeks it develops a thickened nonepithelialized, mildly enhancing wall between the necrosis and the adjacent tissue and only then is then termed WON [20, 26] (Figure 1).
Figure 1. Suggested Algorithm for the Management of Acute Pancreatitis. Abbreviations: See text. Disclaimer: This algorithm is derived generalizations of the literature, with the understanding that there is significant overlap among pathways and no one algorithm can adequately address all patients; diagnostic and treatment decisions should not rely solely on the information presented here.
MRI versus CT
MRI is often reserved for detection of choledocholithiasis not visualized on PPCT images and to delineate pancreatic ductal anatomy. Although PPCT is still the workhorse of imaging for NP in most institutions, some groups prefer MRI [36]. In addition to the ability to visualize ducts with high resolution, other advantages of MRI include its lack of ionizing radiation, which is especially useful for those patients who are pregnant or need serial, surveillance imaging [20, 26, 36]. In patients with very poor renal function, who can therefore receive neither MRI nor CT contrast agents (typically with GFR <35 mL/min), noncontrast- enhanced MRI provides better resolution and structure definition than non-contrast-enhanced CT.
Using Ranson's criteria as a gold standard, Arvanitakis et al. [27] compared CT and MRI in the detection of areas of hypoperfusion compatible with pancreatic necrosis, and found MRI to have a higher sensitivity (83% vs 78%) and specificity (91% vs 86%). However, MRI is more prone to motion artifact, and many patients with NP are unable to breath-hold adequately. The rapid and widespread availability, excellent image quality, and resistance to motion artifact make PPCT an overall better modality for NP.
Infection
Approximately one third of patients with pancreatic necrosis will develop infection, which is associated with a markedly increased risk of mortality (Table 2) [3]. Gram-negative bacteria are the usual culprit but a trend towards increasing infections with Gram-positive and multiresistant organisms has been observed [37, 38]. The development of infection should be suspected by a newonset fever, tachycardia and increasing leukocytosis. The distinction of sterile from infected necrosis is difficult but it very important as it greatly affects the patient’s prognosis and management. The presence of gas on imaging studies is highly suggestive of infection but it is only present in a minority of cases, need not be present for infection to be present [20, 39, 40]. CT-guided percutaneous aspiration for Gram stain and culture has been recommended when infected necrosis is suspected but has begun to fall out of favor in general, given that in the majority of cases infection may be diagnosed based on clinical and imaging signs alone, with aspiration reserved specifically for those cases where the diagnosis in unclear or the result will clearly change management [16, 20, 41]. Prophylactic antibiotic use in the presence of pancreatic necrosis has been shown (but with only level III data) not to change the incidence of infection or mortality and is not recommended as prophylaxis [3, 42], with the possible exception (as discussed below) of imipenem or meropenem, for which a significant decrease in pancreatic infection was found in a 2010 Cochrane review [43], as discussed below.
Bleeding
Hemorrhage can develop in patients with NP especially in the late phase; it is estimated to occur in 1% to 6.2% of patients with acute pancreatitis [44, 45]. The bleeding may occur within the gastrointestinal tract, the peritoneal cavity, fluid collections or in the pancreatic parenchyma.
It usually results from enzymatic degradation of local vessels in the peripancreatic tissues and the development of pseudoaneurysm [46]. Bleeding will often manifest as sudden deterioration in hemodynamics with drop in hemoglobin, the development of a new mass, or bloody output from drains placed in the pancreatic bed. Angiography with embolization should be considered as the initial line of therapy and surgery should be reserved for refractory cases [47]. Another cause of gastrointestinal bleeding is pancreatitis is variceal bleeding associated with splenic vein thrombosis, which itself results from pancreatitis and leads to in left-sided portal hypertension. Bleeding occurs in 4% to 12.6% of patients and splenectomy is rarely indicated [48, 49].
Abdominal Compartment Syndrome
The development of abdominal compartment syndrome (ACS) is associated with a mortality of 49% and a morbidity ranging from 17% to 90% [50]. Surgical decompression has been employed in the form of standard midline laparotomy, bilateral subcostal peritoneum-sparing laparotomy [51], and subcutaneous, skin-sparing, linea alba fasciotomy [52]. In a retrospective study, Mentula et al. reported that early abdominal decompression is associated with improved renal and respiratory function and reduced mortality [53]. A recent systematic review suggested that strong data is still lacking regarding the management of ACS in the setting of acute pancreatitis [50].
Pancreatic Duct Disruption and Stricture Formation
Disconnected-duct syndrome (DDS) is a form of pancreatic duct disruption (PDD) that results from necrosis of a long, central part of the pancreas with preservation of viable tissue in the tail of the pancreas (Figure 1). This upstream orphaned remnant, however, is in discontinuity with the gastrointestinal tract such that any of the sequelae of a PDD may result, such as formation of a pancreatic or peripancreatic collection, pancreatic ascites, pancreatic effusion, or pancreatic fistula [54]. Surgery in the form of pancreatectomy or internal drainage of the cyst can be reserved for patients who fail nonoperative therapy [55, 56], although in our experience, DDS generally requires operation.
In cases without DDS, nonoperative management of PDD is often possible, and is best performed with a multifaceted approach, such as what has recently been termed [57] the SEALANTS approach (Somatostatin, External drainage, ALternative nutrition, Antacids, Nil-per-os, Total parenteral nutrition, and a Stent in the pancreatic duct). Such an approach requires multidisciplinary cooperation [54], and can be useful in avoiding operation in otherwise refractory cases.
Pancreatic-Duct Strictures
Pancreatic-duct strictures can develop after an episode of NP and may later result in fibrosis and scaring, which is associated with recurrent pancreatitis [58].
It is important to assess the severity of acute pancreatitis, as mild acute attacks carry a risk of mortality less than 1% [59] while SAP with necrosis has a mortality rate of 10% to 30% in many recent series but as high as >60% is some subgroups, such as those with extensive and/or infected necrosis [3-5, 7, 34, 60]. As discussed above, MAP is defined by a lack of organ failure, or local or systemic complications; by contrast, both MSAP and SAP are defined by the presence of local or systemic complications, such as those described above, and/or by the presence of organ failure, which is transient (<48 hours) in MSAP, and persistent (>48 hours) in SAP [16, 35].
Although there are many illness-severity scales in general, and many definitions of organ failure in particular, the authors of the revised Atlanta classification chose the Marshall scoring system [61] due to its simplicity, accuracy, and universal applicability, allowing comparison of patients across the world, and comparison of any particular patient's course at various time points in the disease course SAP [16, 35]. Essentially, it assesses the three most commonly affected organ systems, respiratory, renal, and cardiovascular (Table 3). A score of 2 or more for any organ system defines failure.

Single-parameter assessment of severity has been investigated as well. Many such tests, including hematocrit, IL-6, C-reactive protein (CRP), and procalcitonin have been studied with variable success [62-65]. For example, A CRP level of 150 ng/L within 48 hours was found to be 86% sensitive, but only poorly specific (46%) for pancreatic necrosis [64]. The utilization of procalcitonin as predictor or infection and organ failure has been increasingly applied in critical care clinical practice, including prediction of organ failure and infection of pancreatic necrosis in particular: At 24 hours from the onset of symptoms a level greater than 0.4 ng/mL was 97% sensitive and 73% specific for predicting organ failure in a prospective Finnish study [66]. At 48 hours, a level of 1.8 ng/mL was found to be 92% sensitive and specific predicting infected necrosis [62]. At 48-96 hours in a prospective international multicenter study, a level >3.8 ng/mL predicted both infected pancreatic necrosis and organ dysfunction with sensitivity and specificity of 79% and 93% respectively [67]. Procalcitonin may be the best single predictor of morbidity, mortality, and infection in NP.
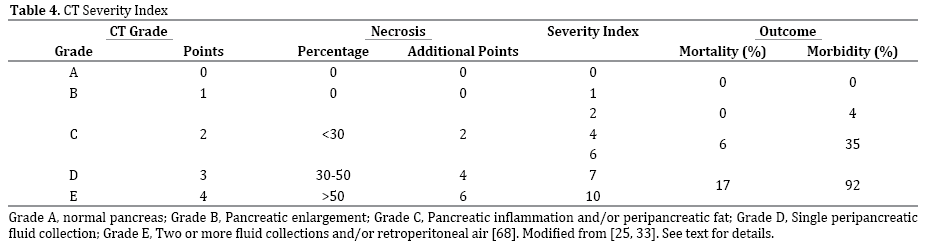
The CT severity index (CTSI) has been popularized by Balthazar, et al. [24, 25, 33, 68]. Building on a simple A-to-E grading system published in 1985 [68], they expanded the system by assigning zero to four points for grades A to E, plus additional points for necrosis (Table 4). Using this system, there was significant correlation with morbidity and mortality [25].
Acute Phase: First 1-7 Days
An algorithm for the management of acute pancreatitis, with a focus on necrotizing pancreatitis, is shown in Figure 1. The use of early, aggressive fluid resuscitation is the cornerstone of therapy during the first 48 hours of the acute phase. Unfortunately, the data on optimal fluid type and rate are not particularly robust [69]. There are, however, level-I data supporting the choice of Ringer’s lactate over normal saline as the initial resuscitation fluid of choice [70]. A reasonable goal is to aim for improvement in systemic inflammatory response and urine output without producing severe pulmonary edema or ACS. The optimal rate of fluid resuscitation to achieve this goal is likely 250-500 mL per hour assuming cardiovascular, renal, or pulmonary comorbidities are not prohibitive [69, 71].
The role of antibiotics in the acute phase of NP has long been debated as a prophylactic measure to prevent conversion of sterile necrosis to infected necrosis. Because the risk of infection of pancreatic necrosis increases with the extent of necrosis (Table 2), many practitioners have elected for antibiotic prophylaxis especially if the extent of necrosis is >30%, but a recent Cochrane review, evaluating 7 trials with 404 patients comparing prophylaxis with no antibiotics, found no significant difference in rates of mortality or infection of necrosis [43]. When subgroups were analyzed according to type of antibiotic, however, those patients receiving imipenem or meropenem had significantly lower rates of infection in the necrosis (RR 0.34, 95% CI 0.13 to 0.84) [44].
Octreotide has been investigated as an agent with the potential benefit in acute pancreatitis but its role is controversial. Although there are some level-I data supporting its use [72, 73], a meta-analysis of randomized controlled trials found no significant benefit [74].
The mode of delivery of early nutrition has been reasonably well studied. Petrov et al. [75] randomized patients predicted to have SAP to either total parenteral nutrition via central venous catheter and total enteral nutrition via radiologically placed nasojejunal feeding tube, both initiated within 72 hours, and found that the incidence of infected pancreatic necrosis was significantly lower in the enterally fed group. Given that the stomach is much more accessible than the jejunum for direct enteral feeds, and given that there is a theoretic concern that enteral feeding should be postduodenal to avoid stimulating a severely injured pancreas, Singh et al. performed a randomized noninferiority trial comparing gastric and jejunal feeding, finding equivalent rates of infectious complications, pain with refeeding, intestinal permeability, and endotoxemia [76].
Invasive interventions in this phase are relatively contraindicated, with a few exceptions. For example, patients who develop ACS, as described above, may necessitate decompressive laparotomy if nonoperative measures fail, including improving abdominal wall compliance with sedation and analgesia, emptying the stomach with a properly maintained nasogastric tube [77], draining intraabdominal collections of fluid, avoiding excessive fluid resuscitation as allowed by the SAP and, optimizing end-organ perfusion [78]. In this and any phase of the disease, consideration should be given for transfer to a high-volume pancreatic center if resources and expertise at the presenting hospital are of questionable adequacy.
Delayed Phase: Weeks 2-6 and Beyond
This phase is marked by ongoing inflammation, local or systemic complications, organ failure, or any combination of them. By definition, therefore, essentially all patients is this phase have SAP. During this time, the extent and geography of pancreatic necrosis becomes more clearly defined on CT. Systemic inflammatory response syndrome and multiorgan failure in this stage are managed with aggressive supportive care and nutritional support. Patients who developed transient organ failure that has resolved or is improving generally tolerate advancement of oral intake and proceed toward discharge. Indeed such patients represent an increasing proportion of those with NP. A recent multi-institution prospective cohort study in the Netherlands found that nearly two-thirds of patients with pancreatic necrosis are successfully management without an invasive intervention [79, 80]. Patients not requiring intervention typically have necrosis that is either sterile, <30% in extent, or both.
When, however, infected pancreatic necrosis is suspected, intervention is generally indicated, and begins with immediate administration of pancreas-penetrating antibiotics, such as imipenem or meropenem (Figure 1). While open surgical debridement used to be considered the standard of care [6], surgical intervention should be avoided in these patients unless absolutely necessary, given that mortality rates as high as 65% have been reported with early operation in cases of NP [5, 6, 28]. Yet, infected fluid collections warrant drainage and this is frequently accomplished via percutaneous catheters, given that this minimally invasive intervention is well tolerated in even critically ill patients with organ failure. However, there are several reports of successful treatment of even infected pancreatic necrosis with antibiotics alone [81, 82].
Catheter-Based Therapy
In 2010, the publication of the PANTER trial [79, 80] popularized the "step-up" approach, in which the first "step" is the percutaneous or endoscopic (transgastric) catheter drainage. Patients on this trial followed a strict protocol such that, if after 72 hours there was no significant clinical improvement, or if the position of drain(s) was inadequate, then the next step was a second drainage procedure. If after an additional 72 hours, there was still no clinical improvement then the next step was video-assisted retroperitoneal débridement (VARD), followed by open necrosectomy only if needed after VARD. This and many other similar reports of minimally invasive treatment [83, 84] have been associated with a paradigm shift away from open pancreatic debridement. However, because VARD is not available in every high-volume pancreatic surgery center, progression to standard open operation is also reasonable depending on availability of resources and expertise (Figure 1).
The basis of this paradigm shifting is the concept that the retroperitoneum containing pancreatic necrosis in need of debridement may be accessed by following the tracks of percutaneous drains and that debridement of this space is possible using large-bore trocars. Laparoscopic cameras and instruments are utilized through the one large trocar, or alternatively several trocars can be placed into the peripancreatic fluid collection.
Factors that have predicted success of percutaneous drainage alone include a decrease in size of peripancreatic fluid collections by >75% in the first 1-2 weeks [83]. Factors predicting failure following VARD include the presence of a centromedian collection extending into the root of the small-bowel mesentery, which was the most common reason for conversion to open necrosectomy in a multicenter, prospective phase 2 study of VARD [83].
A recent meta-analysis by Mouli et al. [85] analyzed data from 8 studies, with 324 patients with infected pancreatic necrosis undergoing nonoperative management (intensive care, antibiotics, and, and nutritional support, with or without drainage) and an additional 4 studies of 157 patients also undergoing nonoperative management, but all undergoing percutaneous drainage. With the understanding that some degree of publication, patientselection, or other bias likely exists, the authors concluded that a nonoperative approach is successful in up to 64% of patient with infected necrosis. Even when catheter-based therapies succeed in avoiding operation for the NP itself, operation may sometimes be required for the pancreatic fistula that may result, as a controlled pancreatic fistula, from percutaneous therapy. However, in our experience, these rarely require operation if an aggressive nonoperative, multifaceted approach such as SEALANTS is taken [57].
Endoscopic Management
Endoscopic management of fluid-filled lesions is different than that of lesions containing solid material, such as WON. Therefore differentiating between them is imperative. The fluid-filled collections, typically PPs, are generally very amenable to EUS-guided cystogastrostomy, assuming a mature and tenacious apposition between the stomach and the PP. Typically, the EUS is performed from within the stomach and a needle is placed into the cyst, the tract is dilated, and then several plastic stents or a single selfexpanding metal stent is placed through the common wall to allow drainage of fluid from the PP into the stomach.
Patients with WON, by contrast, are treated differently. While simple drainage as for PP is occasionally effective, formal endoscopic necrosectomy is often required. It is performed by using cautery to create a cystogastrostomy large enough to allow advancing the endoscope into the WON and performing debridement using endoscopic instruments. Following debridement, stents are placed to maintain patency of the tract. Often a nasobiliary drain is left in the WON and used during subsequent days or weeks to irrigate and further debride.
Unfortunately, the literature on endoscopic treatment of NP is rather limited. Several retrospective series have reviewed recent experience with endoscopic drainage and/or necrosectomy for patients with pancreatic necrosis, concluding in general that is safe and effective for select patients [86-89]. Van Brunschot et al. performed a systematic review of papers published through June 2013, screening 581 papers, only 14 of which (455 patients) fulfilled the eligibility criteria [90]. The quality was in general was poor, with most studies not reporting the severity of disease prior to intervention [90]. A mean of four (range 1–23) endoscopies were performed and treatment was deeded successful in 81% of patients, with a low mortality (6%) and acceptable complication rate (36%) [90]. One recent small but randomized controlled trial from the Dutch Pancreatitis Study Group evaluated patients undergoing endoscopic versus open necrosectomy and found that the endoscopically treated patients had a reduced pro-inflammatory response as measured by IL-6 levels and an improved composite clinical endpoint (including organ failure; procedural complications such as bleeding, enterocutaneous fistula, and pancreatic fistula; and long-term complications, such as new-onset diabetes, use of pancreatic enzymes, and persisting fluid collections) as compared patients randomized to open necrosectomy [91].
Dual-Modality Drainage
The use of combined endoscopic and percutaneous drainage was popularized by the Virginia Mason group [92, 93], although others had previously reported successful use of the combined technique [94]. The Virginia Mason group has recently reported their long-term results, evaluating 117 patient all of whom underwent dual modality drainage (DMD) for symptomatic and infected WON [91]. Of 103 patients completing therapy, follow-up was 750 days and no patient underwent open necrosectomy, despite a 64% incidence of DDS in the population. Half of all patient experiencing a recurrent collection, however, had DDS, but these were managed in all but one patient nonoperatively [92].
While the data from this study are compelling, DMD has not been adequately compared to open necrosectomy, as patients undergoing open drainage were excluded a priori. Therefore the population reported likely had less severe disease, and was generally healthier than their counterparts undergoing open operation. However, the mean CTSI of 7.8, the 60% of patients staying in the ICU, and the 64% of patients with DDS all show that this was not a population with mild disease [92].
Open Surgical Debridement
When less invasive approaches have failed, or are not otherwise appropriate or possible, then open debridement may be necessary. A traditional open approach to necrosectomy may be performed via either a subcostal or a midline incision, with entry into the lesser sac to allow debridement generally achieved through gastrocolic ligament, through the transverse mesocolon, or through the stomach directly. Although there are many approaches and many techniques, one of the most important aspects of open debridement is simply timing. When performed early, operation for pancreatic necrosis is associated with a prohibitively high morbidity and mortality and therefore the goal is to wait as long as possible, but at least 3-6 weeks [3, 95-97]. Operation early in the disease process is indicated only for very specific reasons, such as bleeding, ACS, or ischemic bowel [95]. When patients undergo early operation as a last resort, because of failure of intensive care management with multiple organ failure, the mortality is approximately 100% [97]. When delay is possible, intervention may still be warranted due to significant ongoing illness with failure to thrive. The additional time allows demarcation of the necrotic from the viable pancreas such that careful blunt debridement is rewarded with excellent extirpation of the necrosis with minimal to no bleeding.
After necrosectomy, drains are laid in and around the necrosectomy bed, and these may be placed to suction and may be on occasion be used for postoperative lavage. Following both necrosectomy percutaneous drainage, a controlled pancreatic fistula may result, but will generally close with nonoperative management, such as the SEALANTS approach, as described above [57]. In cases of DDS (Figure 2), the orphaned pancreatic tail must be provided with a new route to the gastrointestinal tract. In these cases the transgastric approach is an especially attractive route for debridement, as a durable operative cystogastrostomy between the posterior wall of the stomach and the rind of the WON provides this route. When open debridement is better approached elsewhere, such as via the transverse mesocolon, then pancreaticoenteric continuity may be re-established via a cystojejunostomy. Depending on the nutritional tolerance of the patient, some form of feeding tube may be indicated at the time of open necrosectomy.
Figure 2. CT image showing walled-off necrosis (WON) causing pancreaticduct disruption (PDD). A young, otherwise healthy male with moderately severe acute alcoholic pancreatitis developed an acute necrotic collection (ANC) in the mid-body of the pancreas that matured into WON (asterisk) over the subsequent month. The patient had recurrent symptoms and failure to thrive due to failure of the secretions from the viable pancreatic tail (arrows) to traverse the area of pancreatic necrosis (asterisk) to reach the viable pancreatic head (solid arrowheads). Percutaneous and endoscopic efforts failed, and he underwent successful open debridement, with a Roux-en-Y cystojejunostomy. After an uncomplicated recovery, he is well nearly 2 years later.
In most patients, a single necrosectomy is required, especially in the current era of deferred operation [79, 80]. Similarly, although morbidity and mortality vary over a very large range in the literature, depending predominantly on the severity of disease and comorbidities and on the era in which the debridement occurred, in recent well controlled series such as the PANTER trial, the risk of postoperative bleeding and fistula are as low (22% and 22%) with open debridement as with as with the minimally invasive "stepup" approach (16% and 14%, NS). Mortality was also statistically equivalent (16% versus 19%) [79, 80].
Acute pancreatitis has a remarkably wide range of severity, from clinically negligible to precipitously fatal despite any intervention. Distinguishing APFC and PP from ANC and WON is important and relies of the use of PPCT. Initial appropriately aggressive resuscitation is essential. Progression to multiorgan failure can occur rapidly and portends a life-threatening course. Antibiotics should be withheld in most cases of noninfected necrosis, but when used, imipenem or meropenem are the agents of choice. Early enteral feeding should be encouraged. Intervention for infected pancreatic necrosis should be based on a minimally invasive approach, akin to the "step-up" approach. In all cases of necrotizing SAP, a multidisciplinary approach is needed, using endoscopic techniques, percutaneous drainage, and then, if needed, VARD. Open surgery should be reserved for failure of less invasive techniques, but when utilized it must be within the skill-set of the treating providers and the resources of the hospital.
Authors declare to have no conflict of interest.