Original Article - (2017) Volume 18, Issue 5
1Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
2Division of Hepatobilliary, Department of Internal Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
3Division of Metabolic Endocrinology, Department of Internal Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
4Clinical Epidemiology Unit, Department of Internal Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia
Received July 13th, 2017 - Accepted September 04th, 2017
Context Understanding of non-alcoholic fatty pancreas disease and its clinical impact needs to be continuously improved as non-alcoholic fatty pancreas disease might allegedly develop into chronic pancreatitis and further leads to pancreatic cancer. Non-alcoholic fatty pancreas disease is strongly associated with type 2 diabetes mellitus and long-term type 2 diabetes mellitus is associated with a 2.0-fold increase in the risk of pancreatic cancer. The proportion of non-alcoholic fatty pancreas disease and its associated factors in type 2 diabetes mellitus population has not been well investigated. Objective To investigate the proportion of non-alcoholic fatty pancreas disease and its associated factors in type 2 diabetes mellitus population. Methods This was a cross-sectional study in adult type 2 diabetes mellitus patients who visited Diabetes Clinic, Cipto Mangunkusumo Hospital. Information about age, sex, duration of diabetes, comorbidities, medication, waist circumference, lipid profile and HbA1C were collected. Abdominal ultrasonography was performed on each subject to diagnose non-alcoholic fatty pancreas disease and non-alcoholic fatty liver disease. Association of non-alcoholic fatty pancreas disease with age, sex, duration of diabetes, hypertension, non-alcoholic fatty liver disease, triglyceride and HbA1C were examined. Study Results From total of 171 type 2 diabetes mellitus patients in this study, the proportion of non-alcoholic fatty pancreas disease was 48.5% (95%CI=41.2 to 55.9%). Univariate analysis showed significant differences between non-alcoholic fatty pancreas disease (NAFPD) and non-NAFPD group regarding proportion of non-alcoholic fatty liver disease (PR=1.96; 95%CI=1.41-2.74; p<0.001) and hypertriglyceridemia (PR=1.38; 95%CI=1.02-1.86; p=0.042). On multivariate analysis older age (OR=2.15; 95%CI=1.10-4.23), non-alcoholic fatty liver disease (OR=3.65; 95%CI=1.90-6.99), and hypertriglyceridemia (OR=2.03; 95%CI=1.02-4.05) showed significant association with non-alcoholic fatty pancreas disease. Conclusion The proportion of non-alcoholic fatty pancreas disease in type 2 diabetes mellitus population is 48.5%. Older age, non-alcoholic fatty liver disease and hypertriglyceridemia were associated factors of non-alcoholic fatty pancreas disease in type 2 diabetes mellitus patient.
Diabetes Mellitus, Type 2; Pancreas
BP blood pressure; NAFLD non-alcoholic fatty liver disease; NAFPD non-alcoholic fatty pancreas disease; T2DM type 2 diabetes mellitus; WC waist circumference
Nonalcoholic fatty pancreas disease (NAFPD) is an excessive lipid accumulation in the pancreas in the absence of significant alcohol intake [1]. It is usually an incidental finding during transabdominal ultrasound examination and its clinical significance is still poorly understood. Prevalence of NAFPD has been reported in Asia as well as in Western countries. In Taiwan, Wang et al. reported that 16% of Chinese population had fatty pancreas [2]. In USA, Sepe et al. reported as high as 27.8% of the patients who underwent EUS evaluation had NAFPD [3]. In Indonesia, which represents the biggest Southeast Asian country, the prevalence of NAFPD in the medical check-up population was 35% [4].
Previous studies had shown that NAFPD is associated with T2DM [5, 6, 7, 8, 9, 10, 11]. As a consequence of insulin resistance, pancreatic beta cells will produce more insulin. Beta cells apoptosis will occur as it were exhausted. Then, this empty area will be replaced by adipose tissue [9]. Moreover, hyperglycemia via malonyl-CoA inhibits beta oxidation of fatty acids in the cell mitochondria. These unoxidized fatty acids will accumulate as intracellular triglycerides and develop into NAFPD [12]. It will further decrease beta cell function in T2DM patients [13, 14].
Pancreatic cancer is still the most lethal cancer in the world. The known risk factors for pancreatic cancer include tobacco exposure, longstanding diabetes mellitus, obesity, advanced age, exposure to benzenes, family history, and chronic pancreatitis [15, 16]. NAFPD may allegedly develop into chronic pancreatitis and further leads to pancreatic cancer [17, 18, 19, 20], and facilitates its dissemination [21]. Patients with type 2 DM have a 2-fold increase in the risk of pancreatic cancer [22, 23, 24]. Therefore, T2DM patients with NAFPD should be considered for pancreatic cancer screening and surveillance.
Factors which are known to be associated with NAFPD in general population include male, age over 60 years, hypertension, fasting blood glucose, triglycerides, body mass index, central obesity and nonalcoholic fatty liver disease (NAFLD) [2, 3, 6, 8, 9, 10, 25, 26]. However, previous studies provided inconsistent results regarding the association of NAFPD with age [3], sex [2, 25], hypertension [2, 25], hypertriglyceridemia [25], and NAFLD [9, 10]. These inconsistent results may be due to difference in diagnostic methods, small sample size in some studies and its retrospective design. The aim of our study was to prospectively determine the prevalence of NAFPD in T2DM population at Cipto Mangunkusumo Hospital (RSCM) Jakarta and to determine the associations of NAFPD with age, sex, duration of diabetes, presence of NAFLD, hypertension, hypertriglyceridemia, and HbA1C level.
Study Design and Subject
This was a cross-sectional study in adult T2DM patients (age of 18 or older) who visited diabetes outpatient clinic at Cipto Mangunkusumo National Referral Hospital between January and February 2017. Subjects were recruited consecutively. Subjects with alcohol consumption ≥20 gram per day in the past year; chronic pancreatitis; or nonvisualized pancreas on ultrasound were excluded.
Sample Size
By using the formula to count a single proportion in a population, the minimum sample size to detect the proportion of NAFPD in T2DM population was 80. While by using the formula to detect difference in two unpaired proportions, the minimum sample size to define the association of various factors with NAFPD in T2DM patients was 160.
Data Collection
Data collection includes age, sex, and duration of DM, comorbid, alcohol consumption, and medication used. Each subject’s waist circumference (WC) and blood pressure (BP) were measured. The WC (to the nearest 0.1 cm) was performed at the end of normal expiration on bare skin midway between the lower rib margin and the iliac crest. Central obesity was defined as WC ≥90 cm in male and ≥80 cm in female. Two blood pressure readings separated by intervals of at least 5 minutes were performed after the subject took a minimum of 5 minutes rest and were taken in a sitting position with an appropriate-sized cuff wrapped around the right upper arm using calibrated digital tensimeter (Omron HEM 7221, Japan). Hypertension was defined as on blood pressure lowering drug(s), or systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mm Hg according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
After an overnight 12-hour fast, all subjects underwent blood test for serum triglyceride and HbA1C levels. HbA1C was measured by immunoturbidimetry method and serum triglyceride was measured by glycerol-3-phosphate oxidase phenol aminophenazone method, both of them using automatic clinical chemistry analyzer (Roche, Cobas 501, Germany).
Diagnosis of Fatty Pancreas
All subjects underwent transabdominal ultrasonography in the hepatobilliary division by a junior consultant and re-evaluated by a senior consultant who was blind to the subjects’ information. The ultrasound was done with high-resolution ultrasonography (GE Logic P6, US) using a convex 3.5-MHz transducer, followed the ultrasonography protocol that had been made before. The protocol stated that problems with bowel gas overlying the pancreas can be overcome by some ways, include deep inspiration or expiration; distend the abdomen against the probe (ask the patient to push their stomach out as if they are pregnant); give the patient an oral waterload (2-3 glasses of water is used as a window to look through when it is in the stomach and duodenum); and scan with the patient erect if needed.
Diagnostic criteria for fatty pancreas is an increase echogenicity of the pancreatic body parenchymal over that of the kidney [2, 6, 27]. Since pancreas could not be visualized in the same window with kidney, the examiner compared the echogenicity between liver and right kidney first, then compared the differences between liver and pancreatic echogenicity to obtain an pancreatorenal echo contrast. Increased deposition of fat that infiltrated along the pancreatic septa, has been shown to be a major determining factor of pancreatic echogenicity in one study evaluating pancreatic echogenicity and its correlation with the morphologic appearance of the pancreas in computed tomography [28]. Therefore, it is reasonable to conclude that the “fatty pancreas” found on ultrasound examination is associated with increased fat accumulation pathologically.
Ethics
The research protocol was approved by the Institutional Review Board of Cipto Mangunkusumo Hospital and this study was carried out without any linkage of personal information for all participants. We obtained written informed consent the patients for publication of this report.
Statistical Analysis
All subjects were classified into either the NAFPD or the non–NAFPD group as dependent variable and were divided by age (<60 or ≥60 years-old), sex (male or female), duration of diabetes (<5 or ≥5 years), absence or presence of NAFLD and hypertension, HbA1C levels (<7.0 or ≥7.0) and serum triglycerides (<150 m/dL or ≥150 mg/ dL) as independent variables. Chi Square test was used for bivariate analysis and binary logistic regression was used for multivariate analysis. A p value <0.05 was considered statistically significant. All statistical analyses were performed with SPSS 21.0.
From 191 participants enrolled in the study, 171 subjects were suitable for the data analysis (Figure 1). The characteristics of all study subjects is shown in Table 1.
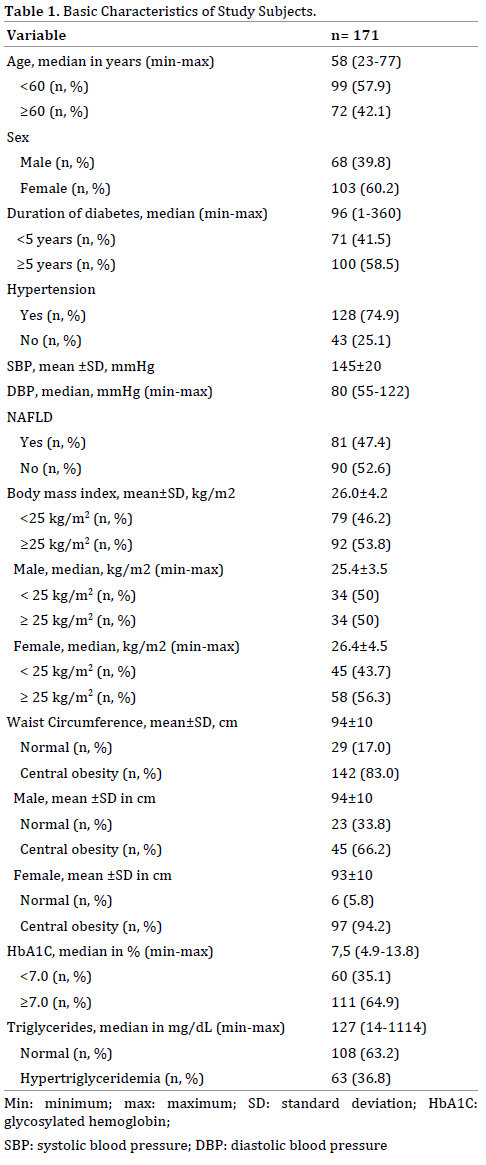
NAFPD was found in 83 (48.5%; 95%CI=41.2-55.9%) subjects, consist of 34 (41%) male and 49 (59%) female. The prevalence of NAFPD is more frequent with increasing age (Table 2). Concurrence of NAFPD and NAFLD on abdominal ultrasound was found in 53 patients (31%).
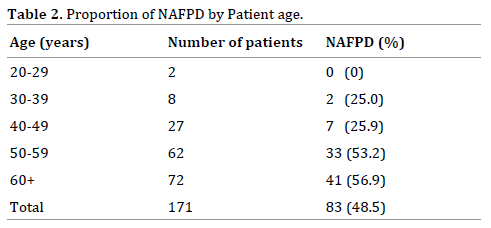
Factors associated with NAFPD on bivariate analysis were NAFLD (PR=1.96; 95%CI=1.41-2.74; p<0.001) and hypertriglyceridemia (PR=1.38; 95%CI=1.02-1.86; p=0.042) (Table 3).
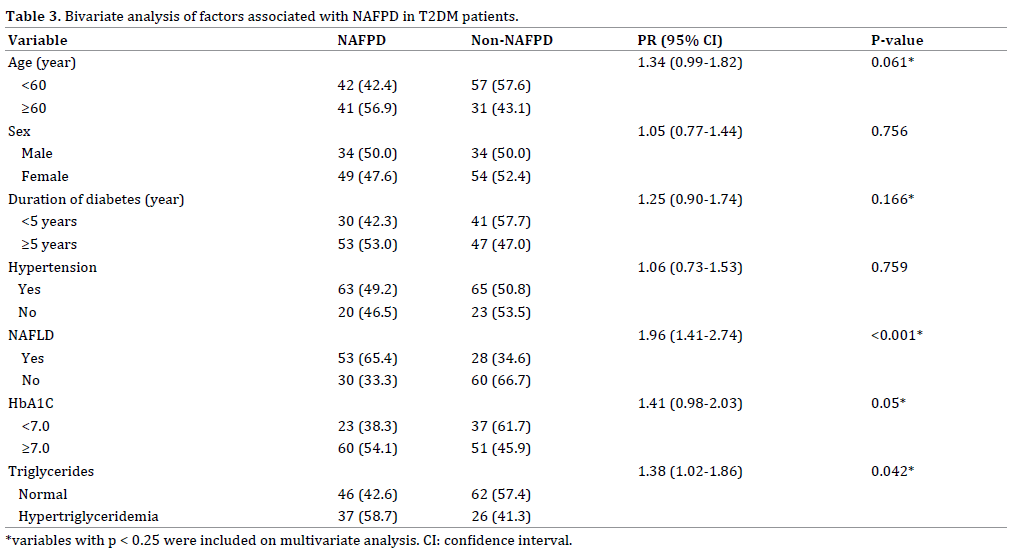
Variables with a p value of less than 0.25 (*) ie age, duration of diabetes, NAFLD, HbA1C and triglycerides were included in multivariate analysis. On stepwise binary logistic regression analysis, factors associated with NAFPD in T2DM patients were NAFLD (OR=3.65; 95%CI=1.90- 6.99), age ≥60 years (OR=2.15; 95%CI=1.10-4.23) and hypertriglyceridemia (OR=2.03; 95%CI=1.02-4.05).
To our knowledge, this is the first prospective study aimed to find the proportion of NAFPD in T2DM population and its associated factors. Our study demonstrated the big proportion of NAFPD in T2DM patients. Recent meta-analysis also showed that there was a significant relationship between NAFPD and T2DM (RR 2.08; 95%CI=1.44-3; p <0.001) [29].
Fatty replacement on pancreas was an inevitable aging process [20, 27, 30]. This was confirmed by our finding that NAFPD was more frequent with increasing age. Other studies using cut off of 60 year-old age [26] and 35 yearold age [4] also showed an association of NAFPD with age. (OR=2.87; 95%CI=1.54-5.37 and OR=4.01; 95%CI=2.82- 5.70). Therefore, older age is considered as an important risk factor of NAFPD.
On the contrary to the previous studies [4, 26], our study showed no association of male gender and NAFPD. It was hypothesized that men are at higher risk to develop NAFPD because they had more visceral (abdominal) fat deposition while women had more subcutaneous (glutealfemoral) lipid deposition [26, 31]. However, in our study there were more centrally obese women (94.4%) than men (66.2%). Majority of female subjects in this study were at monopausal age (above 50 years-old). It had been known that in menopausal woman, fat distribution changed from a gluteo-femoral pattern into a centrally dominant (viseral) pattern due to hormonal changes [31]. Distribution of T2DM patients by age in other countries might be different from ours, so it’s not generalizable to all T2DM patients.
In this study, we found no significant association of NAFPD with duration of diabetes and HbA1C. It may be due to the 2-way relationship between NAFPD and T2DM. NAFPD may occur long time before the onset of type 2 diabetes. This was supported by a study which revealed that pancreatic fat deposition had already been found in the prediabetic phase before the onset of T2DM [32]. Our findings also suggested that insulin resistance play a more important role in the development of NAFPD compared to hyperglycemia.
Hypertension which was known to be a risk factor of NAFPD in previous studies [2, 4, 26] was also not associated with NAFPD in our study subject. This can be explained by the fact that insulin resistance had occurred in T2DM patients long time before they was diagnosed [33, 34, 35], then metabolic syndrome parameters such as hypertension might be found in T2DM patients either with or without NAFPD. Therefore, hypertension which in the general population was a risk factor of NAFPD could not be used as a predictor of NAFPD in T2DM patients.
Consistent with the findings from earlier studies [2, 3, 4], hypertriglyceridemia and NAFLD were also associated with NAFPD in our study. In the state of insulin resistance, excessive free fatty acids [36] were brought and accumulated into various visceral organs including the liver (NAFLD) and pancreas (NAFPD) [37]. Our finding indicated that NAFPD and NAFLD may had the same risk factors leading to fat accumulation in both organs. Thus, the presence of NAFLD may be a predictor of NAFPD in T2DM patients.
This study has several limitations. Owing to its cross-sectional design, we could not determine causal relationship between NAFPD and the variables studied. But this study has given a new insight of metabolic factors associated with NAFPD, which should be considered as a new risk factor for pancreatic cancer development. Furthermore, the pathophysiology of NAFPD is still not fully understood so that other factors that may play a role could be not included in this study. Diagnosis of fatty pancreas in this work was made with transabdominal ultrasound examinations without any histologic confirmation. However, doing pancreatic biopsy in a healthy subject is unethical. The major drawbacks of ultrasound include operator dependency and insensitivity with regard to small amounts of fat. However, it has been proven as a reliable, reproducible and non-invasive screening tool for NAFPD in previous studies [2, 4, 6, 8, 38, 39, 40]. Zhou et al. (n=1190) randomly selected 50 people diagnosed with NAFPD by an abdominal ultrasound to undergo MRI examination and found consistent results [39]. Therefore we still use abdominal ultrasound in this study. Finally, we did not use grading system to assess pancreatico-renal echo contrast. Sepe et al. [3] define the degree of fatty pancreas by using 3 criteria include pancreatic echogenicity, clarity of the parenchyma and pancreatic duct margins. But, to our knowledge, this only can be done by using endoscopic ultrasound not by trans-abdominal ultrasound.
Basic characteristics of our study population resembles that of T2DM patients in 18 secondary and tertiary health care centers in Indonesia [41], and also in community [42], so it is generalizable to all patients with type 2 diabetes in Indonesia.
The proportion of NAFPD in T2DM patients in Cipto Mangunkusumo National Referral Hospital was 48.5%. Presence of NAFLD, hypertriglyceridemia and older age were factors associated with NAFPD in T2DM patients. Screening of NAFPD should be considered to be performed on T2DM patients with those conditions. A prospective longitudinal study is needed to reveal the natural course of NAFPD in T2DM population and its unknown risk factors. We also suggested that greater number of patients included in further study by using abdominal tomography to better evaluate the fat infiltrative pancreatic disease.
We thank Hepatobilliary Division Junior Consultants for their sincerely help and Mrs. Utami S. for helpful advice.
We declare that we have no conflict of interests.