Research Article - (2023) Volume 9, Issue 4
Non-Phthalate Plasticizer/Poly (Vinyl Chloride) Compound for Sustainably Based on Biomaterials Using Glycerol from Biodiesel Process
Aya Soliman1,
Abbas Anwar1,
N.S.Yousef1,
Hossam Anwar1 and
Ashraf Morsy1,2*
1Department of Petrochemicals, Pharos University, Alexandria, Egypt
2Materials Science Department, Institute of Graduate Studies& Research, Alexandria University, Egypt
*Correspondence:
Ashraf Morsy,
Materials Science Department, Institute of Graduate Studies& Research, Alexandria University,
Egypt,
Email:
Received: 26-Nov-2022, Manuscript No. IPBMBJ-22-15195;
Editor assigned: 28-Nov-2022, Pre QC No. IPBMBJ-22-15195 (PQ);
Reviewed: 12-Dec-2022, QC No. IPBMBJ-22-15195;
Revised: 22-Feb-2023, Manuscript No. IPBMBJ-22-15195 (R);
Published:
01-Mar-2023, DOI: 10.36648/2471-8084.9.4.33
Abstract
The phthalate replacement trend the globe began when the European Union put a ban on Dioctyl Phthalate (DOP) and other. This trend will continue as environmental and sustainability concerns increase. These polymers, made from renewable sourced (plant based) raw material, as bio based development plasticizers to gain some functionality. At the same time, the biodiesel sector produces an excess glycerol, prompting scientists to look for new uses for this material. The main goal is to create a non-phthalate plasticizer for Poly Vinyl Chloride (PVC) utilizing crude glycol from the biodiesel process, corn Starch (S), sunflower oil, Citric acid (C) and Benzoic acid (B). Glycerol diacetomonolaurate plasticizer will be developed utilizing glycerol from the biodiesel process. Scanning Electron Microscopy (SEM) and Fourier Transform Infrared spectroscopy (FTIR) will be used to examine the plasticizer production, universal testing equipment will be used to determine the mechanical properties of the plasticizer PVC compound, Thermal Gravimetric Analysis (TGA) and the experimental values will be compared with phthalate plasticizer PVC compound. The plasticizing performances of the sample of non-phthalate contain SCB rivaled that of (DOP) phthalate, from this study suggesting that they have the potential to replace phthalate in soft PVC materials.
Keywords
Phtahalates; Plasticizers; Non-phathalete; Eco-friendly; Sustainable; Poly Vinyl
Chloride (PVC)
Introduction
Plasticizers are frequently utilized as additives in the polymer
industry. The major function of these compounds as
phthalates to increase polymer flexibility and processability by
lowering the second order transition temperature, also known
as the glass transition temperature (Tg). Phthalates are a
group of chemicals that can be used as an addition in plastics to control the material's performance. Polymers are softened
with them to make them more flexible and durable.
Phthalates are sometimes used to aid the molding process by
decreasing polymer melting points. Plasticization is essential
for most polymers to be treated particularly true for Poly Vinyl
Chloride (PVC). As worries about environmental
contamination, toxic effect, and consequences on human
health and safety get more and more significant, polymers and polymer additives derived from biomaterials are gaining
in interest. Bio-plasticizers have also been put to the test to
see if they can perform better in terms of functionality than
phthalate-based plasticizers and non-toxicity during
metabolism. The advantages of bio-plasticizers contribute to
over standard phthalate plasticizers, when it especially true
comes to flame retardants and plasticizers. Starch is both
biodegradable and inexpensive, but it is also extremely
hydrophilic. It has already been intensively investigated as a
major raw material for environmental and medicinal
applications. Native starch is partially crystalline and occurs as
separate granules with 20 percent-45 percent
crystallinity. Because there are many hydrogen bonds
between the starch macromolecules, which limit their
motion, native starch is difficult to process. Thermo Plastic
Starch (TPS) is made by destroying granules in the
presence of plasticizers under controlled conditions. Much
research and effort has gone into the combination of
plasticizers with nanoparticles before placing them into the
polymer (CaCO3, SiO2). While this helps to reduce migration to
some level, the amount of plasticizers that remain escape
constitutes a health risk. This research focuses on the
fundamentals of plasticizers, stressing the drawbacks of
traditional phthalates based plasticizers, as well as the
necessity for environmentally friendly bio based
plasticizers it also examines numerous biomass sources in
order to find the finest available biomass sources. Starch is a
natural renewable polysaccharide that has been considered as
a possible raw material for bio plastics manufacture. However,
it must undergo extensive change in order to lose its natural
structure and become thermoplastic. Thermo Plastic Starch
(TPS) is a remarkable material for producing non-durable
products. Citric acid could be a suitable choice for expanding
the variety of properties that can be accessed. In comparison
to other chemicals used for derivatization, Citric Acid (CA) is
considered nutritionally safe. It's a harmless body metabolic
product that's previously been approved by the Food and
Drug Administration (FDA) for human usage. Citric acid and
starch could form an ester bond. Esterification could occur
between the carboxyl groups on citric acid and the hydroxyl
groups on starch. Even if there is no ester link between citric
acid and starch, citric acid has been shown to establish
significant hydrogen bond interactions with starch, even
stronger than glycerol. The thermoplastic starch's heat and
water sensitivity was then enhanced, and retro gradation was
prevented. Trans-esterification is a step in the biodiesel
production process that produces glycerol as a byproduct.
Because it is an unprocessed raw material that must be
purified for its many uses, the utilization of this glycerol is
limited. Several studies have looked into the potential
applications of unprocessed glycerol. Mekonnen T, et al.,
studies the bio-based plastics and impact on the performance
of these materials such as poly (lactic acid). Howell BA, et al.,
used non-phthalate plasticizers derived from well-defined
glycerol/adipic acid hyper branched polyesters has been
produced. Adipic acid and glycerol are two low toxicity
renewable biomaterial's. These materials are thermally stable,
completely compatible with a PVC matrix, provide effective
plasticization at acceptable levels, have a low migratory potential. Lakeev SN, et al., provide details on new plasticizers
made from terephthalic acid are being researched as a
possible replacement for the hazardous dioctyl phthalate. The
properties of non-phthalate plasticizers based on benzene,
toluene, naphthalene, cyclohexane, and norbornene have
been investigated. The utilization of plasticizers made from
renewable vegetable raw materials such citrates, succinates,
triglycerides, fatty acid esters. The objectives of this work is to
produce glycerol as a byproduct of the biodiesel
manufacturing process, and then to make plasticizers (nonphthalate
plasticizers) from glycerol, citric acid, benzoic acid,
sunflower oil, miscibility with polymer polyvinyl chloride at the
best mixing ratio. On the other hand, we looked into how
composition affects PVC plasticization. In addition to prepare
films containing different plasticizer and varying amounts of
plasticizer were created [1-6].
Materials and Methods
Materials
Alpha chemical (Mumbai, India) provided citric acid (99% purity) and benzoic acid, corn starch, the acetic acid, was obtained from Merck. Dioctyl Phthalate (DOP) was obtained from Aldrich chem. The sunflower oil glycerol was obtained as a byproduct from biodiesel production. Low molecular weight poly (vinyl chloride) was purchased Alexandria based Egyptian petrochemical business.
Preparation of Glycerol from Biodiesel Process
At the same time, heat the 800 ml vegetable oil to 55°C, and then add the methanol NaOH solution to the heated oil until the methanol is immiscible with the vegetable oil, and a layer forms. This is why it's important to stir vigorously to avoid any problems forming, which would cause the reaction to fail. At 500°C, the mixture must be stirred for 30 minutes. "Liberating the fatty acid from the glycerol backbone" is the transesterification reaction. Stirring is stopped after 30 minutes, and the mixture is allowed to settle for at least an hour. The solution will split into two layers: The glycerol in the lower layer and the biodiesel in the upper layer.
Preparation of Plasticizer
With the addition of distilled water, the water content of corn starch was increased to 20% (wet base). Glycerol and sunflower oil were mixed first, and then corn starch was blended using the GH-100Y high speed mixer. When the citric acids and benzoic acids were melted, they were first combined with a mixture of glycerol, sunflower oil, and corn starch. The mixture was sealed and stored overnight. Table 1 lists the many samples prepared and their components. The melt blending technique took 13 minutes in the Haake Rheomix (Thermal Electron Co., USA) at 130°C and 80 revolutions per minute [7-12].
| |
Starch |
Glycerol |
Citric acid |
Benzoic acid |
Sunflower oil |
DOP |
| Sample (1) (SGCBSu) |
20 |
20 |
20 |
20 |
20 |
|
| Sample (2) (SGCB) |
25 |
25 |
25 |
25 |
0 |
|
| Sample (3) (SCB) |
33,33 |
0 |
33,33 |
33,33 |
0 |
|
| Sample (4) (DOP) |
0 |
0 |
0 |
0 |
0 |
100 |
Table 1: Samples composition proportions (Wt%).
Mixing Procedure
The following is an example of a common experimental procedure. Mixing was done with an internal mixer and a two roll mixing mill to prepare the master batch for final mixing four different plasticizers with polyvinyl chloride content were added to the mixer, as shown in Table 1. The compounds were then cured in a molding press (Carver, WMV50H, and USA). Plasticizer and PVC weight percent ratio (25:100). We assume the compound, combination that we made is homogeneous with the PVC because we stirred it under 160°C: PVC and NON Ph (1), PVC and NON Ph (2), PVC and NON Ph (3), PVC and Ph (DOP) (4).
Film Production
Prepare flow sheet of PVC film with different type of plasticizer (phathaleta and nonphathalte as shown in Figure 1.

Figure 1: Flow sheet of PVC film with different type of plasticizer.
Characterization
Fourier Transforms Infrared (FTIR): FTIR spectroscopy is a potent qualitative and semi quantitative analytical method. Organic molecules absorb electromagnetic radiation with a frequency range of around 100 cm-1-4000 cm-1 and transform it into molecular vibration energies: Stretching and deformation (bending). The relative masses of the atoms, the shape of the atoms in the molecule, and the force constants of the bonds between atoms determine the frequency of absorption. Characterizing film is done using incident radiation FTIR (Agilent Technologies, Cary 630). The spectra are recorded in the 400 cm-1-4000 cm-1 wavenumber range.
X- Ray Diffraction (XRD)
To assess the degree of crystallinity in the produced disc, X-ray diffraction scans were performed at room temperature with a (X-ray 7000 Schimadzu-Japan) in the Bragge angle (2 ) range of 10°C to 80°C. The X-ray source was a Cu target with 30 KV and 30 mA settings with a scan speed of 4 degrees per minute.
Scanning Electron Microscopy
Scanning electron microscopy was used to examine the surface morphology of the sample. The samples were made as follows: The dried film was sliced and placed on a brass. The film was then sputter coated with a tiny layer of gold using a (JSM IT200, LaB6) microscope.
Thermo Gravimetric Analysis (TGA)
The samples were examined using a TGA model (Linseis STA PT 1000). This research is required in order to identify the degradation temperature. The sample was heated in a silica crucible at a constant rate of 10 k/min from 24°C to 700°C.
Mechanical Tests
Film of PVC had their tensile strength and percent elongation at break average values evaluated at room temperature using an Instron 3382 (100 KN) universal testing machine with a crosshead speed of 10 mm/min, according to ASTM D882-028.
Results and Discussion
Structure Investigation
Infrared spectroscopy was used to analyse the chemical
structure of plasticizer (SGCBSu) revealed a growing
transmission band of the CA starch chain. The stretching
vibration of 'C–O' in 'C–O–C' is attributed to the peak at 1023
cm-1 (Figure 2). The height of the peaks at 1024 cm-1 dropped
as the (C) content increased. The reference peak was again
chosen as the stretching, vibration peak of 'C–O' in the 'C–O–
H' group at 1289 cm-1. The drop in the 'C–O–C' caused the
peak height to fall. The reason for this could be that the
greater the (C) level, the more glycosidic linkages are acid
hydrolyzed. The following characteristics were also noticed:
Two broad bands separated by 3255 cm-1 and 3048 cm-1.
Between 1669 cm-1 and 1298 cm-1, there are three distinct,
powerful bands: The CO peak at 1669 cm-1 is most likely a
coalescence peak formed by the ester bond and carboxyl CO
groups in citric acid, because all of the carboxyl groups are
unlikely to be esterified. The peak at 1709 cm-1, which is
attributable to the C=O stretching, vibration in carboxyl
groups, was only identified as a reference in the FTIR spectra
of pure CA. The presence of ester linkages was demonstrated
by the shift in the position of the CO peak in the sample
(1,2,3), and the esterification reaction between starch and CA
occurred during the process of melt blending.
We despite the fact that the system contains some esterified
carboxyl groups, the number of esterified carboxyl groups
rose as the number of ester linkages grew. As a result, the rise
in peak height at 1669 cm-1 can also be attributed to the
increase in ester bonds. When a 'C–O–H' on the starch
reacted with (C) molecule to produce a 'C–O–C,' the starch
received a new 'C–O–H' from the CA, and the total quantity of
'C–O' bonds in 'C–O–H' groups were regarded to be the
smartest that a broad peak of C=O stretching, vibration could
not occur. Heights of the other peaks were addressed. The
esterification occurred during melt mixing, according to FTIR
results, and the (C) % increased. Inter and intermolecular
hydrogen bonds can be disrupted by the esterified citric acids
that were linked to the starch chains. The free carboxyl groups
connected to esterified citric acid may also help to promote
starch solubility and avoid crystallization. Although there is
some carboxyl groups that have not been esterified (Figure 2).
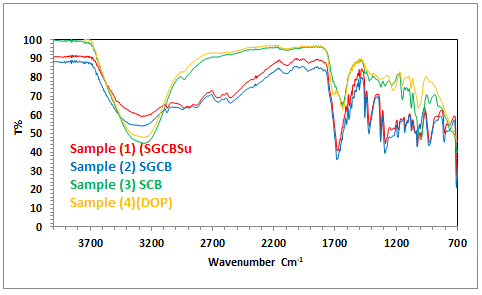
Figure 2: FTIR spectra of different plasticizer.
The FTIR spectra of PVC are shown in Figure 2. The CH group
stretching is responsible for the band at 2898 cm-1, while the
CH aliphatic carbon stretch is responsible for the band at 1405
cm-1. In addition, the absorbed water's H-C-H bending group
occurred at 1266 cm-1. The C-H bending peak was at 1,380
cm-1, and the C-C stretching bands were at 1066 cm-1, while
the bands at 710 cm-1 corresponded to the stretching modes
of the CL-C single bond vinyl group. In addition, the FTIR
spectrum of PVC and (SGCBSu) as a plasticizer (Figure 3).
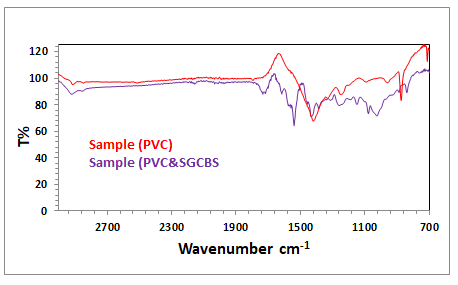
Figure 3: FTIR spectra of PVC with plasticizer (SGCBSu).
Morphological Properties of Mixture PVC and
Plasticizer
The inner and exterior aggregation morphology of the PVC
grains was examined using a Scanning Electron Microscope
(SEM). After being axed in conductive glue, a reasonable
number of sample grains were coated with a thin layer of gold
using a gold sputter coater under vacuum. A scanning
electron microscope was used to analyze the aggregation
morphology of the PVC grains. SEM is a useful instrument for
testing the compatibility of various components of polymeric
materials. This method can detect the various interfaces and
separation phases inside the polymeric matrix, which reflect
mechanical and thermal stability as well as ionic conductivity.
SEM images also reveal the shape and size of particles.
According to SEM images, the non-irradiated PVC (blank) and
PVC/additive mixes had clean and neat surfaces with a high
degree of uniformity. SEM images of the irradiated PVC are
shown in Figure 4.
The granular microstructures of PVC and SGCBS (1) may be
seen in the SEM image. This could be due to the fact that it
acts as a neutral scavenger via H-bonding and a host guest
interaction controlled by the size effect and plasticizer
dispersion. Figure 4 shows SEM images of the interior of a
standard PVC grain and the interior of those grains formed in
the presence of PVC and SGCB (2). While PVC resin, totally
fused primary particle agglomerates produced by
conventional suspension polymerization, PVC grains formed in
the presence of PVC and SGCB (2) are made up of a large
number of distinct, fine primary particles. They eventually
clumped together to form primary particles on the droplet's
surface and became a part of the skin. PVC and SCB showed
an increase in cell population density and a reduction in the
size of the miracles. Due to the addition of a vibration force
field, the sample of formulation number showed a stronger
orientation. Figure 4 shows how the plasticizer (DOP) affects
the cell shape and density of PVC microcellular plastic. PVC
and DOP and PVC and SCB morphological produced the
smolset microstructure, with the lowest cell population
density and homogeneous and compatible. Figure 4 shows
that PVC and SGCB and PVC and SGCBSu are more the biggest
cells and partial miscibility with PVC [13-18].

Figure 4: SEM images of surface for PVC and SGCBSu (1), PVC
and SGCB (2), PVC and SCB (3), PVC and DOP (4).
X-Ray Diffracion (XRD)
X-Ray Diffraction (XRD) is a useful technique for determining
the interior structural organization of solids. It can be used to
distinguish between solids that are crystalline, amorphous, or
semi crystalline. Figure 5 shows the XRD pattern, which used
to determine whether a crystalline phase present in the
structure. The XRD curves of PVC and SGCBSu with a small
amount of CA changed insignificantly as the hydrogen bonds
decreased, indicating that the motional freedom of starch
chains in amorphous regions increased. The internal plasticizer
is the CA that has reacted with starch, while the residual CA in
the mixes acts as an exterior plasticizer. Additionally,
esterification can alter the groups on starch chains. On the
starch molecules, additional groups (carboxyl and ester
groups) were created, which can provide potential reactive
locations for cross linking modification. The role of the mixture
PVC and SGCBS can be studied using full range analysis of the
filled samples, which exhibits a diffraction peak at roughly
2ϴ=18°. The homogeneous distribution of the polymeric
matrix is revealed by examining the XRD diffraction patterns of
PVC films containing various types of plasticizer while PVC has
an amorphous phase in general. At the location depicted in Figure 5 (1-4) ascribed to CA, benzwic acid, many peaks with
lower intensities emerge. It was also discovered that the
amorphous halos around 2ϴ=26° are less intense in the filled
PVC films than in the pristine PVC films, indicating that the
addition of plasticizer reduces the crystalline phase in PVC
filled samples, in agreement with Rajendran, et al., and
pointing to the formation of multiple phases in the new
material composed of combined semi crystalline and
amorphous phases. The XRD pattern of a polymeric for all
samples shows the formation of a sharp band at about 38°and
the persistence of distinctive amorphous halos around 18° and
25°.
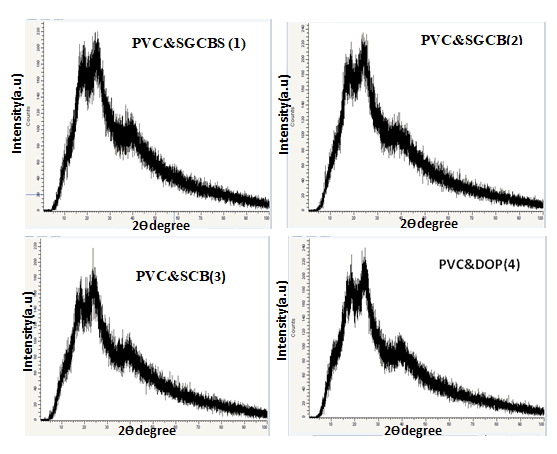
Figure 5: X-Ray diffraction scans of PVC an d different
plasticizer.
Mechanical Properties
The influence of several types of plasticizer on PVC sheet was
investigated using the mechanical properties of the
manufactured flow sheet. The mechanical testing system was
used to determine the tensile strength. Figures 6 shows stress
vs strain curves for (PVC and SGCBS (1), PVC and SGCB (2), PVC
and SCB (3), and PVC and DOP (4), respectively, indicating
that mechanical strength increases in the order specified.
The effective load transfer from PVC and SGCBSu (1) to the
PVC and DOP (4) under tensile stress is responsible for the
rise in modulus, tensile strength, and elongation. The
following obtained data and their assignment can be
summarized in Table 2.
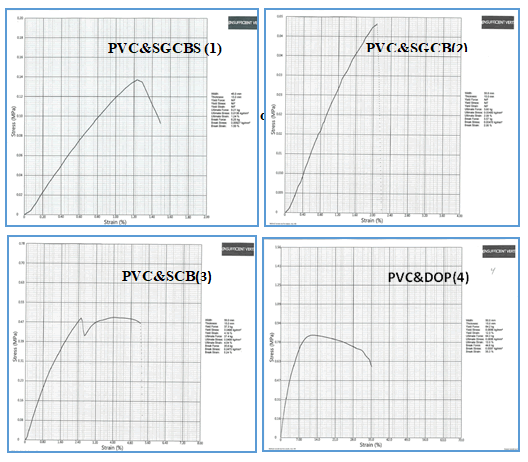
Figure 6: Stress versus strain curves of PVC and plasticizer
DOP
| No |
Width (mm) |
Thickness (mm) |
Ultimate force (Kg) |
Ultimate stress (Kg/mm2) |
Ultimate strain (%) |
Break force (Kg) |
Break stress (Kg/mm2) |
Break strain (%) |
| 1 |
45 |
15 |
9.21 |
0.0136 |
1.24 |
6.25 |
0.00927 |
1.5 |
| 2 |
50 |
15 |
3.6 |
0.0048 |
2.08 |
3.57 |
0.00476 |
2.08 |
| 3 |
50 |
15 |
37.4 |
0.0498 |
4.64 |
35.5 |
0.0475 |
5.24 |
| 4 |
50 |
15 |
64.2 |
0.0856 |
12 |
44.8 |
0.0597 |
35.3 |
Table 2: Mechanical properties data for samples.
Thermo-Gravimetry Analysis
The TGA curves of plasticized PVC by several plasticizer blends
are shown in Figures 7-10. For all formulas, the curves are
similar in shape, and each one shows three unique stages.
The 0.82137 mg weight percent loss (T), which is the initial
weight loss temperature, the maximum degradation
temperature (T1 max and T2 max) (229.7°C), which is the
highest thermal degradation rate, temperature obtained from
the peak of weight loss, the maximum speed of degradation
(S1 max and S2 max), and the 4.6 percent weight loss residue
were chosen as the characteristically thermal parameters. At
around 229°C, a significant amount of HCl was eliminated. It
could be due to the first stage of heat deterioration. As the
process of heat degradation of polymers involves cyclization
and breaking of chains, the 58.55 weight percent loss residue
is attributed to crosslinking of chains containing C=C bonds.
The results reveal that at a greater temperature, the PVC
mixed with SGCBS begins to lose weight than the PVC step at
temperatures above 411°C the third stage of deterioration
begins by intermolecular cyclization of the conjugated
sequences during this stage, thermal breakdown of the plan
sequences yields volatile aromatic and aliphatic chemicals,
leaving a 32.7 weight percent loss residue. The results of
initial weight loss temperature to maximum degradation
temperature (T1 max and T3 max) for all samples for
(Figures 7-10) of PVC mixed with plasticizers SGCBSu, SGCB,
SCB and DOP as the following 62.59%, 76.4%, 75% and 67%
respectively [19-21].
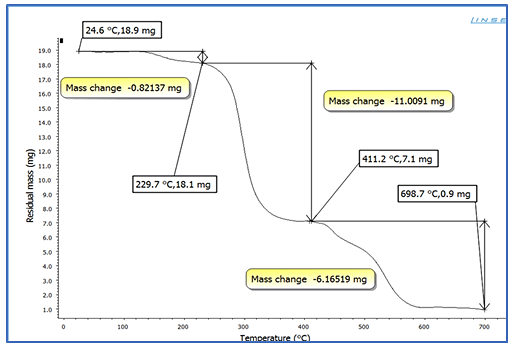
Figure 7: TGA curves for PVC with plasticizer SGCBSu.
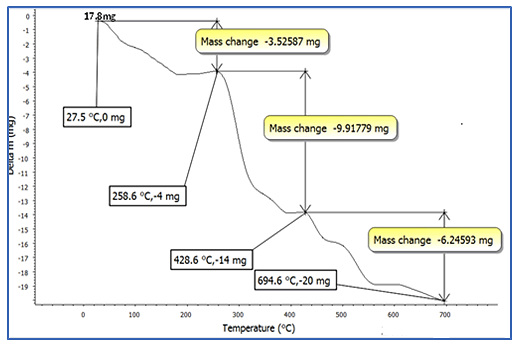
Figure 8: TGA curves for PVC with plasticizer SGCB.
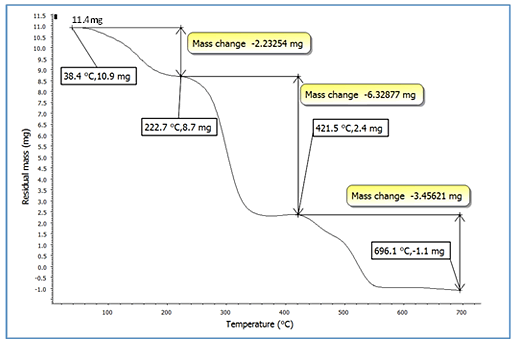
Figure 9: TGA curves for PVC with plasticizer SCB.
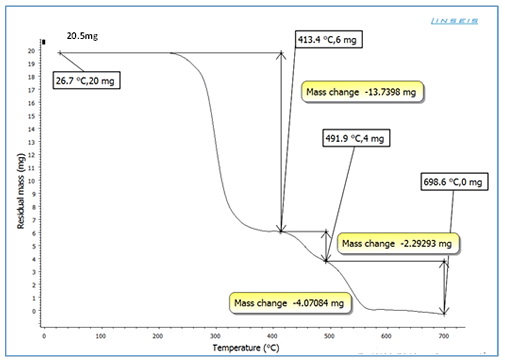
Figure 10: TGA curves for PVC with plasticizer DOP.
Conclusion
In this work were successfully prepared glycerol for the
biodiesel production process as byproduct and preparation of
plasticizers (non-phthalate plasticizer) from prepared glycerol,
citric acid, benzoic acid, sun lower oil, miscibility with polymer
polyvinyl chloride achieving the best mixing ratio. On the
other hand, we studied the in luence of composition on the
plasticization of PVC. Consequently, various PVC ilms
containing different plasticizer and different amounts of
plasticizer were produced by the different type technique. The
morphology of prepared plasticizers (non-phthalate
plasticizer) and structure were con irmed by SEM, X-Ray
diffraction patterns. The morphology, properties of PVC and
SCB ilm were enhanced than the other samples, which
improved the compatibility due to the presence of the
carboxylic groups, ester group in the CA and basic acid. In
addition the mechanical (elongation, modulus of the ilm),
thermal stability properties of PVC and SCB showed very good
behavior than the samples. Therefore, the SCB (non-phthalate
plasticizer are thought to be useful for PVC polymer. In the
present work, two parameters characterize the efficiency of a
plasticizer were controlled by varying amounts of a plasticizer
and adding polymers of different compositions as plasticizer.
Funding
Not applicable.
Human and Animal Participants
This article does not contain any studies involving animals’
studies or human participants performed by any of the
authors.
Data Availability Statement
All data will be available at reasonable request from
corresponding author.
References
- Muobom SS, Umar AMS, Soongseok Y, Brolin AP (2020) A review on plasticizers and eco-friendly bioplasticizers: Biomass sources and market. Int J Eng Res 9(5):1138-1144.
[Google Scholar]
- Wei XF, Kallio KJ, Bruder S, Bellander M, Hedenqvist MS (2019) Plasticizer loss in a complex system (polyamide 12): Kinetics, prediction and its effects on mechanical properties. Polym Degrade Stabi. 169:108985.
[Crossref] [Google Scholar]
- Daniels PH (2009) A brief overview of theories of PVC plasticization and methods used to evaluate PVC‐plasticizer interaction. J vinyl addit Technol. 15(4):219-223.
- Shtarkman BP, Razinskaya IN (1983) Plasticization mechanism and structure of polymers. Acta Polymerica. 34(8):514-520.
[Crossref] [Google Scholar]
- Snejdrova E, Dittrich M (2012) Pharmaceutical applications of plasticized polymers. Recent Advan Plastic. 159, 23-34 (2012).
[Google Scholar]
- Zuraida A, Yusliza Y, Anuar H, Muhaimi RMK (2012) The effect of water and citric acid on sago starch bio-plastics. Int Food Res L. 19(2):715-719.
[Crossref] [Google Scholar]
- Zhang Y, Han JH (2006) Plasticization of pea starch films with monosaccharides and polyols. J Food Sci. 71(6):E253-E261.
[Crossref] [Google Scholar]
- Cha DS, Chinnan MS (2004) Biopolymer based antimicrobial packaging: A review. Crit Rev Food Sci Nutr. 44(4):223-237.
[Crossref] [Google Scholar] [PubMed]
- Krauskopf LG (2003) How about alternatives to phthalate plasticizers? J Vinyl Addit Technol. 9(4):159-171.
[Crossref] [Google Scholar]
- Ambrogi V, Brostow W, Carfagna C, Pannico M, Persico P (2012) Plasticizer migration from cross‐linked flexible PVC: Effects on tribology and hardness. Polym Eng Sci. 52(1):211-217.
[Crossref] [Google Scholar]
- Seligra PG, Jaramillo CM, Fama L, Goyanes S (2016) Data of thermal degradation and dynamic mechanical properties of starch–glycerol based films with citric acid as crosslinking agent. Data Brief. 7:1331-1334.
[Crossref] [Google Scholar]
- Wei XF, Linde E, Hedenqvist MS (2019) Plasticiser loss from plastic or rubber products through diffusion and evaporation. NPJ Mater Degrad. 3(1):1-8.
[Google Scholar]
- Chabrat E, Abdillahi H, Rouilly A, Rigal L (2012) Influence of citric acid and water on thermoplastic wheat flour/poly (lactic acid) blends. I: Thermal, mechanical and morphological properties. Ind Crops Prod. 37(1):238-246.
[Crossref] [Google Scholar]
- Dhoot SN, Freeman BD, Stewart ME (2002) Barrier polymers. Polym Plast Technol Eng. 8(2):155-175.
[Crossref] [Google Scholar]
- Shi R, Zhang Z, Liu Q, Han Y, Zhang L, et al. (2007) Characterization of citric acid/glycerol co-plasticized thermoplastic starch prepared by melt blending. Carbohydr Polym. 69(4):748-755. [Crossref] [Google Scholar] [PubMed]
- Ye J, Liu S, Xiang J, Lei J, Zhou C (2013) Preparation and application of triglyceride plasticizers for poly(vinyl chloride). J Appl Polym Sci. 129(4):1915-1921.
[Crossref] [Google Scholar]
- McCormick K, NiinaKautto (2013) The bioeconomy in Europe: An overview. Sustainability. 5:2589-2608.
[Crossref] [Google Scholar]
- Mekonnen T, Mussone P, Khalil H, Bressler D (2013) Progress in bio-based plastics and plasticizing modifications. J Mater Chem. 1(43):13379-13398.
[Crossref] [Google Scholar]
- Howell BA, Lazar ST (2019) Biobased plasticizers from glycerol/adipic acid hyperbranched poly(ester)s. Ind Eng Chem Res. 58(37):17227-17234.
[Crossref] [Google Scholar]
- Lakeev SN, Maydanova IO, Mullakhmetov RF, Davydova OV (2016) Ester plasticizers for polyvinyl chloride. Rus J Appl Chem. 89(1):1-15.
[Google Scholar]
- Wei XF, Kallio K J, Bruder S, Bellander M, Hedenqvist MS (2019) Plasticizer loss in a complex system (polyamide 12): Kinetics, prediction and its effects on mechanical properties. Polym Degrad Stabil. 169:108985.
[Crossref] [Google Scholar]
Citation: Soliman A, Anwara A, Anwara H, Morsya A (2023) Non-Phthalate Plasticizer/Poly (Vinyl Chloride) Compound for Sustainably Based on Biomaterials Using Glycerol from Biodiesel Process. Biochem Mol Biol J. 9:33.
Copyright: © 2023 Soliman A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.











