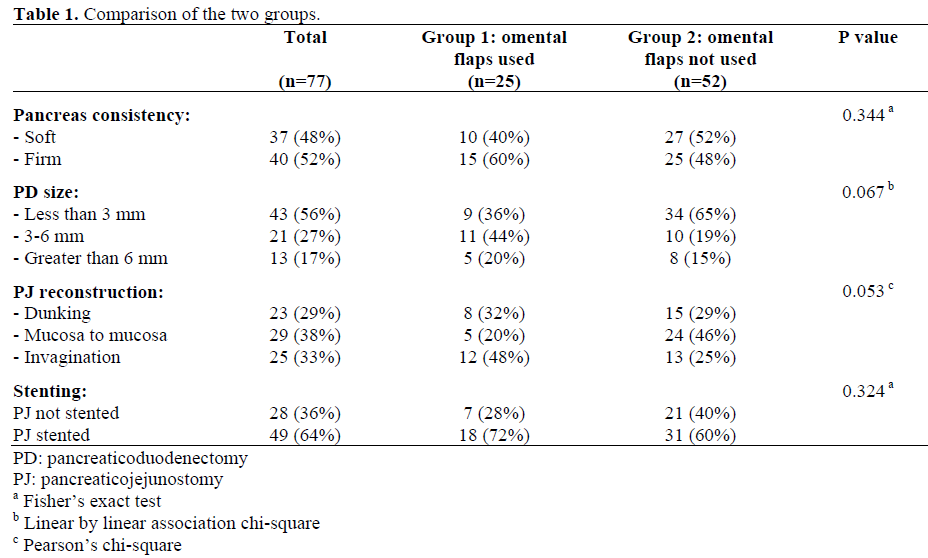
- (2006) Volume 7, Issue 6
Vinay K Kapoor, Ajay Sharma, Anu Behari, Rajneesh K Singh
Department of Surgical Gastroenterology, Sanjay Gandhi Post-Graduate Institute of Medical Sciences. Lucknow, India
Received: 24 May 2004 Accepted: 8 June 2004
Context Pancreaticoduodenectomy continues to have a high morbidity (40-50%). Major complications of pancreaticoduodenectomy include leaks from the pancreatico-jejunostomy and an intra-abdominal bleed from the gastroduodenal artery stump. The omentum has been used for the prevention of anastomotic leaks.Objective The use of omental flaps to prevent a pancreaticojejunostomy leak and bleeding complications from a pancreaticojejunostomy leak after pancreaticoduodenectomy. Patients Seventy-seven patients who under-went a pancreaticoduodenectomy.Interventions Pedicled flaps were made from the greater omentum. One omental flap was wrapped over the pancreaticojejunostomy (separating it from gastroduodenal artery stump) and the second omental flap was wrapped over the duodenojejunostomy.Results Omental flaps were used in 25 patients (Group 1) and a pancreatico-duodenectomy was done without an omental flap in 52 patients (Group 2). None of the 25 patients had any complications related to the omental flap. A pancreaticojejunostomy leak occurred in 4/25 (16%) patients in Group 1 and in 11/52 (21%) patients in Group 2 (P=0.762). None of the pancreatico-jejunostomy leaks in Group 1 was clinically significant. The pancreaticojejunostomy leaks in Group 2 were responsible for intra-abdominal bleeding in 2 patients (1 died) and for intra-abdominal abscess in 5 patients (1 died). Neither of the 2 (8%) deaths in Group 1 was related to a complication from pancreaticojejunostomy. There were 5 (10%) deaths in Group 2 (three following an intra-abdominal bleed, one due to bleeding from the gastrojejunostomy, and one due to sepsis following a pancreaticojejunostomy leak). Thus, there were four patients in Group 2 who died from a pancreaticojejunostomy leak and/or a major vascular bleed vs. none in Group 1 (P=0.298).Conclusion The use of omental flaps is a simple technique for decreasing the risk of major vascular complications related to pancreaticojejunostomy leak following pancreaticoduodenectomy.
Carcinoma, Pancreatic Ductal;
duodenectomy; Pancreaticojejunostomy; Postoperative Complications
GDA: gastroduodenal artery; GJ: gastrojejunostomy; HJ: hepatico-jejunostomy; PD: pancreaticoduodenectomy; PJ: pancreaticojejunostomy; PPPD: pylorus preserving pancreaticoduodenectomy
Pancreatic resection provides the only chance of cure and long term survival for patients with periampullary and pancreatic cancers. Pancreaticoduodenectomy (PD) has become an increasingly safe operation for selected patients with benign and malignant periampullary and pancreatic head disorders. Though mortality has decreased, the cumulative morbidity has remained static over the years at 40-50% [1, 2]. Major complications of PD include a leak from the pancreaticojejunostomy (PJ) and an intra-abdominal bleed from the gastro-duodenal artery (GDA) stump [3]. A PJ leak initiates a subsequent chain of events leading to an intra-abdominal abscess, sepsis and erosion into the adjacent vessels (GDA stump being the most common) [3]. The incidence of a PJ leak increases with the soft texture of the pancreas, the small diameter of the pancreatic duct, and technically difficult anastomoses (pancreas not holding sutures well) [4]. Various methods have been described in the literature to prevent PJ leaks and subsequent complications (e.g. use of somatostatin or octreotide), various types of anastomosis techniques (e.g. duct to mucosa, dunking or invagination) [5], stenting the PJ, and using glue over the PJ. Bleeding is another major complication of PJ. It can be intra-luminal (an anastomotic bleed or a bleed from the cut surface of the pancreas) or intra-abdominal (from one of the adjacent vessels or from the walls of the abscess cavity).
The omentum has an enormous capacity for adhering to traumatized tissues, revascularization, angiogenesis, antibacterial defense, reducing hemorrhage by pressure and activating prothrombin by the rapid conversion of fibrinogen to fibrin [6]. It has traditionally been used for the prevention of post-operative septic complications caused by dead spaces, anastomotic leaks and fistula formation following intestinal anastomoses or perforations, and bronchial leaks after pneumonectomy [6].
We report our experience with the use of omental flaps to protect the PJ and to prevent bleeding complications from a PJ leak after PD.
Between January 1989 and April 2006, a total of 303 PDs were done by 8 consultant surgeons in a 60-bed Surgical Gastroenterology Unit at a tertiary level referral center in Northern India. In 2002 one of the authors (VKK) introduced the use of omental flaps to protect the anastomoses and adjacent vessels with the aim of reducing the complications from PD. The results of the PDs done by three surgeons (VKK, AB, RKS) with and without omental flaps were compared.
A total of 77 PDs were performed: 30 (39%) patients had classic (Whipple’s) PD and 47 (61%) had undergone pylorus-preserving PD (PPPD). Indications for PD were ampullary carcinoma in 59 (77%), duodenal carcinoma in 6 (8%), and cholangiocarcinoma in 7 (9%) patients; other indications were carcinoid tumor, serous cystadenoma, papillary cystadenoma, stromal tumor of the duodenum, and chronic pancreatitis in one patient each (1%). There were 55 (71%) men and 22 (29%) women; the median age was 49 years (range: 25-82 years). Associated comorbidities included diabetes in 9 (12%), cardiovascular disease in 6 (8%) and cirrhosis in one patient (1%).
Surgical Technique
PD was done using the standard technique. The GDA stump was closed by double ligatures of non-absorbable sutures. In PPPD, a duodenal length of 2 cm distal to the pylorus was preserved. All three anastomoses (i.e., PJ, hepaticojejunostomy (HJ), and duodeno-jejunostomy (DJ) or gastro-jejunostomy (GJ), in that sequence) involved a single loop of the jejunum. PJ was done using various techniques (depending on the texture of the gland, the diameter of the pancreatic duct, choice of surgeon) e.g., end-to-side duct to mucosa, end-to-end or end-to-side dunking as described by Sikora and Posner [7] with interrupted sutures of Prolene® (Ethicon Division, Johnson & J Johnson Ltd., Mumbai, India). The PJ was stented in the case of a soft pancreas, undilated duct, pancreas not holding sutures, and difficult anastomoses. HJ was done in end-to-side fashion with interrupted sutures of Vicryl® (Ethicon Division, Johnson & Johnson Ltd., Mumbai, India) in a single layer. A trans-choledochal T-tube was placed to drain the bile; one limb of the T-tube was placed in the jejunal loop towards the PJ to decompress the jejunal loop (Figure 1). A DJ/GJ was done in two layers (outer seromuscular with Prolene®/silk and inner full thickness with Vicryl®). A Ryle’s tube was guided into the efferent limb of the jejunum for enteral feeding. Two large lumen (28F) drainage tubes were placed (one behind the HJ and other behind the PJ), and were brought out through the right flank. A double limb closed suction drain was placed anteriorly with one limb superior and the other inferior to the PJ with the intention of preventing any collection anterior to the PJ (area not drained by posteriorly placed drains).
Figure 1. Schematic representation of pancreaticoduodenectomy with omental flaps and various drains. Inset 1: Crosssection of duodenojejunostomy and the omental flap wrapped over it. Inset 2: Pancreaticojejunostomy and the omental wrap covering the anastomosis and separating it from the gastroduodenal artery stump.
Pedicled omental flaps, preferably two, were made from the greater omentum. Each flap was 3-4 cm wide and was based on an epiploic branch of the gastro-epiploic artery (Figure 2). One omental flap (on the right) was placed in front of the portal vein, behind the PJ (in its bed) before beginning the anastomosis and. was wrapped over the PJ flap was fixed to the pancreas and the jejunum by fine interrupted sutures to keep it in place. The second omental flap (on the left) was used in a similar fashion to wrap over the DJ.
Seru
on postoperative days 4 and 7. A contrast study was done through the PJ stent or T-tube to document an anastomotic (PJ or HJ) leak and a contrast-enhanced computerized tomography (CECT) scan was carried out, whenever indicated.
Data for all patients
post-operative complications (as defined below), were prospectively maintained. The primary endpoint of this study was the incidence of PJ related post-operative complications: i.e., to compare the rate of such postoperative complications in patients who had an omental flap versus those who did not have an omental flap at the time of Surgey.
Definitions Used
A PJ leak was defined
a
amylase activity) fluid in the drain beyond post-operative day 5 or a leak demonstrated on contrast study or CECT. Intra-abdominal abscess was defined as a collection associated with fever and positive culture, requiring either percutaneous or surgical drainage. A bleed was defined as an intra-abdominal bleed if it appeared in the drains or was intra-peritoneal as detected at laparotomy and as an intra-luminal bleed if it appeared in the T-tube, Ryle’s tube or manifested as melena. Deaths occurring within 30 days of the procedure were considered surgical deaths.
This is a reechniques
t
Written consent was obtained from patients undergoing pancreaticoduodenectomy under general anesthesia. However, the technical details of the procedure were left to the choice of the surgeon; therefore, approval by the institutional review committee was not necessary.
The Fisher exacontingency tab
the linear by linear chi squared tests were used to analyze nominal and ordinal discrete data, respectively. P values were considered statistically significant at the 5% level. Statistical analyses were performed by means of the SPSS for Windows, Version 14.0.
Omental flaps anastomoses (Group 1) and PD was done without the use of omental flaps in 52 patients (68%); most of whom had been operated on before 2002 (Group 2). The two groups are compared in Table 1. None of the patients had any complications related to the omental flap. A PJ leak occurred in 4/25 (16%) patients in Group 1 and in 11/52 (21%) patients in Group 2 (P=0.762; Table 2). The PJ leaks in Group 1 were minor and contained leaks (seen at radiographs or diagnosed by high drain fluid amylase); one patient with a PJ leak had an intra-abdominal abscess just beneath the anterior parietal wall requiring repositioning of the drain. None of the leaks in Group 1 was clinically significant, resulted in a complication or required intervention (Figure 3). The PJ leaks in Group 2 were responsible for an intra-abdominal bleed in 2 patients (1 died) and for an intra-abdominal abscess in 5 patients (1 died) (Figures 3 and 4). Three (12%) patients in Group 1 had a bleeding complication but none had a PJ leak as an antecedent cause of the bleed (Figure 3). All three patients with bleeding were re-explored; one was found to have a diffuse oozing from the cavity near the drain, the second had bleeding from the retro-peritoneal operative site raw surface and the third patient bled from an omental vessel injury at the time of drain removal. Two patients (4%) in Group 2 had bleeding complications caused by PJ leak: one patient had a major bleed from the GDA stump requiring angio-embolization and the other had slow ongoing oozing from an abscess cavity in the retro-peritoneum requiring surgical management.
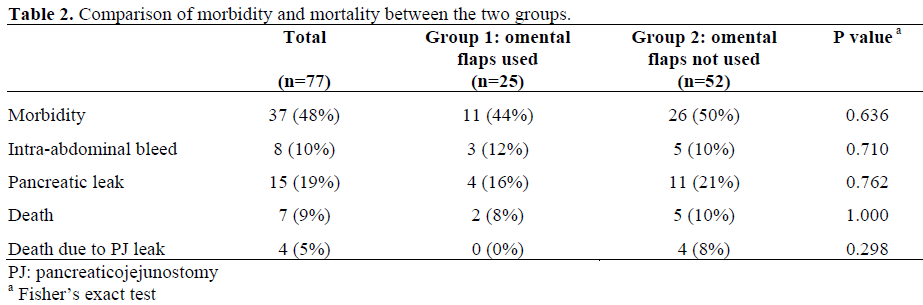
Two (8%) patients died in Group 1: one from myocardial infarction on day 9 a from severe acute post-operative pancreatitis and its complications on day 19. None of the patients in Group 1 died because from a PJ leak and/or a major vascular bleed (Figure 4). There were 5 (10%) deaths in Group 2: three following an intra-abdominal bleed, one due to bleeding from the GJ, and one due to sepsis following a PJ leak. Thus, 4 patients in Group 2 died from a PJ leak and/or a major vascular bleed (Figure 4). No significant difference (P=0.298) was found in the number of patients who died from bleeding between Group 1 (0/25) and Group 2 (4/52; 8%).
PD, which wa
d
now a sufficiently safe procedure due to better surgical techniques and improved postoperative care. Although earlier associated with high mortality rates (30% in 1970s), currently, when done in high volume centers (centers of excellence) or by high volume surgeons, the peri-operative death rates have dramatically decreased to less than 5% over the last two decades [2, 8, 9]. But this steady improvement in mortality has been associated with only modest improvement in morbidity. Data from a multi-institutional analysis from Germany have, of late, confirmed the relatively high risk of complications ranging from 24 to 46% following PD [8]. Major morbidities and mortalities following PD are due to a PJ leak, which has been labeled as “the most feared complication” of PD and still remains a challenge for surgeons. Results of the same multi-institutional study have found PJ leak rates to range from 2 to 28% [8]. A PJ leak leads to peri-pancreatic abscesses which may erode into a nearby vessel which typically bleeds 1 to 3 weeks after surgery [4]. Mortality following pancreatic leak has been reported in up to 40% of patients [10, 11]. Sepsis and bleeding are the two most common causes of death from a PJ leak following a PD, as seen in our earlier experience as well as reported by others [12, 13, 14]. Hence, prevention of a PJ leak is one of the chief factors for reducing morbidity and mortality after PD.
Bleeds after PD include intra-luminal and intra-abdo
bleed is known to occur most commonly as a result of arterial erosion or pseudo-aneurysm formation following PJ leak [3, 15]. Pancreatic trypsin and elastase are responsible for arterial wall digestion, and this is compounded by the presence of bile or infection. Another source of an intra-abdominal bleed can be the walls of the abscess cavity. The reported rates of a post-PD bleed range from 1 to 10% [13, 16]. In spite of attempts at adequate management, a post-PD bleed may culminate in death in 30-50% of cases [14, 16].
The omentum is an ideal structure for protecting an anastom
providing a circumferential soft tissue contact which can act as a plug to prevent early anastomotic leak and provides a source of granulation tissue and neovascularization for healing. Bennett was the first to describe the use of the omentum in 1896 [17]. He used it to plug a perforated gastric ulcer. Since then, the omentum has been used in various experimental and clinical studies with the aim of reducing anastomotic leaks and their complications. Omental flaps have been used to protect various intestinal anastomoses [18, 19]. Adams et al. found that an omental wrap
forms an effective bridge over the anastomotic defect in the first 48 hours and then provides the bulk of granulation tissue aiding in subsequent healing [20]. Omental vessels develop anastomosis with the vessels of adjacent viscera as early as the third postoperative day to aid in anastomotic healing [20]. Ohwada et al. have reported that the omental wrapping of a cervical esophagogastrostomy following radical esophagectomy reduced anastomotic leak [21]. We have used omental flaps to wrap PJ and DJ in PD and found that its use decreased PJ leak rates and avoided a major arterial (GDA stump) bleed even if a PJ leak occurred. Similar results have been shown by other studies in which omental flaps were used to protect the PJ and the vessels near it [22, 23]. Many surgeons may be using omental flaps in PD but very few have reported their results. Seyama et al. reported the use of an omental graft which was placed behind the PJ in 14 patients undergoing PD and concluded that the use of an omental graft prevented pancreatic fistula formation and subsequent intra-abdominal infection and hemorrhage, consequently reducing mortality [22]. Maeda et al. used omental flaps in 100 patients and found that they reduced the incidence of post-operative intra-abdominal hemorrhage, intra-abdominal infection and mortality [23]. However, they found that omental flaps were less effective in controlling PJ leaks; this is probably because they did not use the omental flaps to wrap around the anastomosis as we did; rather, they had kept it only in the bed of PJ anastomoses [23]. We found that, when wrapped around the PJ, an omental flap showed a decreased incidence of PJ leak as compared to the traditional surgical technique, even if without any significant difference, probably due to the low number of subjects studied. Even if a PJ leak also occurred in the new surgical technique evaluated, it was contained by the omental flap, thus preventing erosion into the adjacent vessels, especially the GDA stump, and a major vascular catastrophe. In fact, death due to bleeding did not occur in any of the 25 patients operated on with omental flaps and in 4 of the 52 patients operated on with the traditional technique. It is worth noting that the use of omental flaps has been introduced only recently (since 2002) and patients in whom omental flaps were not used were operated on before 2002. It is possible that the reduction in the incidence of PJ leaks may be due to a learning curve; the prevention of a major vascular catastrophe in the presence of PJ leak, however, can, in our opinion, be attributed to the use of omental flaps.
The use of omental flaps is a simple technique for decreasing the risk of major vasualar
complications related to PJ leak following PD.