Research Article - (2023) Volume 9, Issue 4
Received: 26-Aug-2022, Manuscript No. IPAP-22-14155; Editor assigned: 29-Aug-2022, Pre QC No. IPAP-22-14155 (PQ) ; Reviewed: 13-Sep-2022, QC No. IPAP-22-14155; Revised: 26-Oct-2022, Manuscript No. IPAP-22-14155 (R); Published: 03-Nov-2022
Today we can develop a lot of techniques for treating several illnesses by using different kind of method, by different dosage form; day by day there are new developments in the field of drug delivery system. Now a day’s one of the growing fields in the pharmaceutical area is the preparation of microsponge. Micro sponge gaining popularity due to is less side effect and less expensive and several other advantages over the comparison of the other drug delivery system. Microsponge is now being used as a recent novel technology for control releasing system and as well as target specific medication delivery system fewer side effects and many more reasons for the researchers attracted towards the microsponge drug delivery system. In this article the main focused of our study on method of preparation, classification on the basis of dosage form techniques, compare study of the other category and uses of the microsponge in other field. Microsponge technology provides entrapment of excipients, improving adability, it reducing side effects, reactions, increasing elegance and enhancing formulation flexibility. In cosmetics world skin care, sunscreens and prescribed products were being used currently. The best features of this technique are it has a self-sterilizing activity.
Microsponges; Cosmetics; Topical formulation; Dynamic specialist; MSP (Micro sponge)
In 1987 the first micro sponge innovation was created by the scientist won and the chief licenses were being naming to advanced polymer systems, Inc [1]. This company created up a gigantic number of assortments of the systems and applied those to remedial similarly as OTC and arrangement pharmaceutical things. At present, this entrancing advancement has been endorsed to cardinal health, Inc., for use in topical things [2,3].
Medication can be included in microsponge which is amazingly permeable, porous, springy and round form as a fiddle and dynamic fixings discharge in a proceed from the gigantic pore of microsponge. Microsponge size dimension extents from 5 μm to 2900 μm in breadth and 24 μm fit as a fiddle. Around 250000 pores are open in a microsponge. The pore in microsponge can capture a wide scope of prescription and act like being as a novel bearer to convey the medication to the particular objective or can be utilized to get as a controlled and continued release sample of medication [4].
Microsponges are polymeric in nature that is most commonly used for prolonged tropical action. A wide variety of substances has capacity to entangled due to the fact that microsponge having polymer based microsphere and it easily been incorporated into a different method of formulation. Microsponge are mainly delivered or deigned to a deliver the API efficiency at minimum dose and suitable time period it (API Stands for Active Pharmaceutical Ingredients). The essential intrigue of the microsponge innovation emerges from the trouble experienced with customary details in discharging dynamic fixings over a broadened timeframe, terrible smell, oiliness and skin aggravation [5]. Microsponges are polymeric conveyance frameworks made out of permeable microspheres. They are modest wipe like circular particles with an enormous permeable surface and are accepted to contribute towards decreased reactions, improved dependability, expanded class and upgraded detailing adaptability help in stability enhancement reduced the side effect help in modified the drug release concentration.
Microsponges delivery system are very little, clusters of even numbers of spherical particles of minute size patented polymeric structure including of penetrable microspheres that can entrap a wide degree of dynamic fixings that is:
• Emollients.
• Aroma.
• Oils.
• Sunscreens.
• Threatening to infective, against, disease and are uncommonly suffered and essentially achievable, novel substance that didn't experience the skin, arranged for holding different occasions their weight in skin emissions and can retain skin discharges.
The microsponges size can be changed as a from 5 μm-300 μm in width as indicated by the standard [6]. Being disregarding this the way that the microsponge size may move and a common 25 μm circle can have up to 250000 pores its interior pore structure upto 10 ft long giving a firm pore volume of approx. 1 ml/g this outcome in a huge vault inside each microsponge, which can be stacked with up to its own one of a kind burden. The micro sponge’s particles themselves are unreasonably tremendous to ever be absorbed into the skin and this adds an extra security to these materials in microsponges. Another stability concern is the degradation by bacteria on the drug trapped in the microsponge. Since the size of the pore width is minimal litter than microorganisms, running from 0.007 μm to 0.2 μm, infinitesimal living beings can't pass by this pathways structure of the microsponges. The microsponge framework can stay away from over the highest point of fixings inside the epidermis and the dermis. In this way, the microsponge framework provides a powerful medication without being losing their sufficiency [7].
Properties
• It stable mostly upto pH (1-11) and suitable for temperature (up to 13°C).
• Mostly vehicles are compatible with this microsponge.
• Due to its size it cans self sterilizer.
• High entangled efficiency.
• Easy free flowing.
• Sustained and extended release properties.
• Formulation flexibility.
• Skin can easily absorb in it.
• Upto 6 times of their weight it can absorb the oil.
Why studying of microsponge
• Microsponge helps in improving the patient compliance.
• Microsponge help to Modifying release drug delivery system.
• It improving the bioavailability of drug.
• Microsponge improving the product performances by this.
• It targeting the particurly site of drug.
• Microsponge can help in improving the efficacy in treatment.
• Formulation of drug improved very well.
• It minimize the cost, should be safe, nontoxic, non-allergic, non-irritant.
Types of microsponge on the basis drug delivery system:
• Polymeric drug delivery system ex: Dicyclomine.
• Collagen microsponges: Polylactic co-glycolate.
• Floating microsponge ex: Bupropion HCl (Figures 1-3).
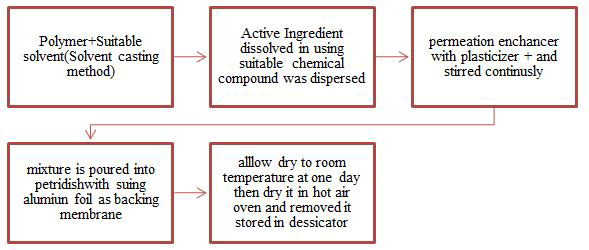
Figure 1: Preparation of microsponge transdermal patches by following some steps.
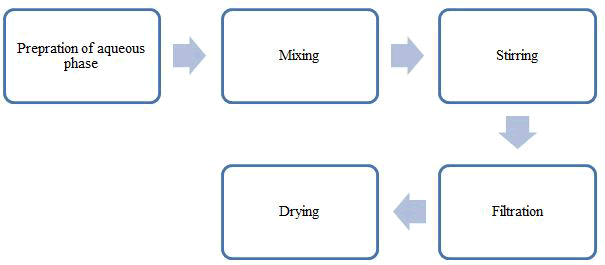
Figure 2: Preparation of microsponges gel.
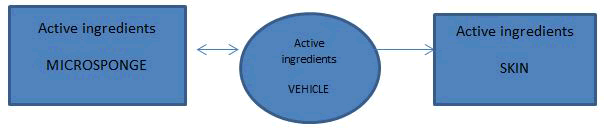
Figure 3: Mechanism of action of the microsponge delivery system.
Material required for microsponges formulation with following characteristics:
• Miscible.
• Water immiscible/slightly soluble.
• Inert.
• Steady in contact with polymerization catalyst.
Categoryvise Analysis of Drugs Over Microsponge Formulation
Antibacterial: Infections, especially skin infections triggered by multiple bacteria constitute an extensive complication that threats the human health. This encourages the researchers to find an alternative for management of skin disorders by encapsulating the antibacterial drugs in novel carrier systems to enhance their efficacy a topical antibiotic used for skin infection is mupirocin. Mupirocin gel was prepared by using a quasi-emulsion solvent diffusion technique of preparation of microsponge. By preparing the microsponge of mupirocin it helps in improving its physical properties and also showed better treatment of primary and secondary skin infection showed enhanced [8,9].
Antipsychotic: Drug used to treat schizophrenia. According to BCS classification, risperidone is a class II compound, poor water solubility so there is a need to develop suitable dosage form researcher with the help of microsponge formulation of risperidone can improve its solubility and efficacy and also help in their physical properties [10].
Anti-emetic: Agents Ondansetron is bitter in taste, its half-life is 5-6 hours, its bioavailability in the body is 60% due to first pass metabolism to overcome these problems we have formulated by some another method and it can give good result but after preparation of microsponges it can be expected to become more improved performance, reduce irritation and shows more extended release properties [11]. Microsponge Ondansetron gel was prepared by using Carbapol 940 and PEG 400. It shows more improving performance in patients and fewer side effects [12].
Antimicrobial activity: This system can additionally help to overcome skin irritation problems [13]. Babchi Oil (BEO) is natural occurring constituent’s use of the BEO loaded microsponge in antimicrobial activity can shows enhanced photostability improved efficacy and payload is good with retartded release [14].
Anti-acne drugs: Now a day’s one of the most common problem is acne, mostly every individual is facing this problem, to overcome from this problem so many pharmaceutical preparation like liposomes, micro emulsions, solid lipid nanoparticles and nanolipid carriers are used but they have some side effects so to overcome from these problem these preparation get converted into microsponges. Microsponges have a vast system and fewer side effects it able to optimize drug activity profile for anti-acne agents [15]. The first line of drug used for antiacne is Benzoyl Peroxide (BPO) due to its bactericidal activity it is superior in other antibiotics, but a side effect of this drug is another system that it causes skin irritation to the individuals. Microsponge preparation of BPO reduces skin irritation, enhance safety without losing its efficacy another drug or second line of drug for acne is Erythromycin but the drug causes gastric irritation, nausea, vomiting, abdominal pain and is easily inactivated in the gastric environment but after preparation of microsponge gel of erythromycin, which exhibited lesser skin irritation, greater skin tolerance and slow release of drug [16,17].
Diabetic foot ulceration: The wound healer cream and gels should not show quick action and good drug release in diabetic patient. Researcher used Atorvastatin calcium as a microsponge gel preparation that showed quick wound and prohealing action in diabetic patient [18].
NSAIDS: Are commonly used drug, selection of COX-2 inhibitor Celecoxib (CXB) is poorly water soluble drug with less oral bioavailability. Many formulation strategies have been produced to enhance total bioavailability of CXB using varied solvent systems, solid dispersion, manipulation of solid state of drug, etc. preparation of microsponge CXB shows that improve its surface along with its bioavailability is also improved [19]. Diclofenac diethylamine is also used in arthritis its oral administration has some disadvantage such as extensive first pass metabolism and maybe it occurs gastrointestinal irritation. Skin lesions is occurring due to the I.V. injection, thus to overcome these problem researchers has develop the microsponge diclofenac diethylamine gel and in their study was found to be promising delivery system, treating rheumatoid arthritis having prolonged release [20].
Anti-inflammatory: For curcumin microsponges, researchers also performed Fourier Transform Infrared spectroscopy (FTIR) studies for the analysis of stability of the product, in addition to the above-mentioned parameters. The FTIR spectra revealed no sign of instability of the drug, suggesting good shelf life of microsponge delivery system [21-25].
Anticholinergic: Gastrointestinal (GI) side effects causing due to dicyclomine tablet of anticholinergic category. The study was done to define that microsponge delivery system that would reduce the GI side effects of that medication [26].
Cardio vascular system: Nicordil first therapeutic drug to shown the hyperpolarize smooth muscles cell membrane best in use of emerging cases and maintenance of high bold pressure but being so it has low oral bioavailability with having a short half-life 1 hr., (hr. stands for hour) so it recommended to the patient used 2 to 4 times a day. The researcher uses this drug to make a microsponge of nicordil after being an microsponge nicrodil is shows improve in oral bioavailability with improving mechanically strong tablets and novel once daily tablets of nicrodil microsponges with controlled release [27]. Another drug is Candesartan Cilexetil (CC) due to its low solubility and a slow dissolution rate researcher study the microsponge of CC an in its study it show that the microsponge the CC drug improve its bioavailability and show dissolution rate [28,29].
Sun cream: Oxybenzone sun cream mostly have to be found that in their formulation it causing the skin irritation. By preparing its microsponge loaded gel help for improving its efficacy and prolonging retention of drug with less permeation, toxicity and improves its sun protection factor [30].
In oral medication these pores are too small as a result not able to be attacked by microscopic particles and the extra increment in the surface area for solubilization and therefore significantly improvement the rate of dissolution [31,32].
In future Microsponges based oral care products like tooth paste and mouth washes can be developed due to its extended release properties of volatile active ingredients which attract customer by enhancing period of “fresh feel”. Even it may be useful to formulate colour cosmetics like lipsticks and rouge because colours which are used in can be entrapped in microsponges to retain long lasting and hence it can provide high elegant product [33].
Method of Preparation
Liquid-Liquid suspension polymerization: In liquid-liquid systems, by suspension polymerization method the microsponge are prepared (Figure 4). In this technique the mixing of two immiscible monomers are dissolving along with excipients in a suitable solvent. In next step, solution of monomer is taken and dispersed in the phase i.e., aqueous phase containing surfactants (example cellulose) to facilitate development of suspension. Then by increment in temperature or by addition of catalyst its polymerization is activated [34-36]. So the polymerization process results in formation of polymeric microsponge, the formed microsponge then separated from liquid medium using suitable technique [37].
Figure 4: Preparation by liquid-liquid suspension method of microsponge.
Quasi emulsion solvent diffusion: The porous microsponges are also organized by another method by using quasi emulsion solvent diffusion technique [37]. This method involves mainly two steps. In this technique, first step a polymer and drug is dissolving in suitable volatile solvent. The solvent is then poured into aqueous phase containing suitable stabilizer with continues stirring for at least for 2 hrs. After entire evaporation of volatile solvent, the shaped microsponge separated by way of suitable method [38]. The product was washed and dried using suitable methods (Figure 5) [39,40].
Figure 5: By quasi emulsion solvent diffusion method of preparation of microsponges
Others methods by which microsponges are prepared:
• Polymerization (Liquid-liquid suspension).
• Fibroblast development factor incorpated in a collagen wipe sheet solvent diffusion (Quasi-emulsion).
• Water in oil emulsion solvent diffusion.
• Oil in oil emulsion solvent diffusion.
• Addition of pyrogen.
• Lyophilization.
• Vibrating orifice aerosol generator method.
• Ultrasound assisted production.
• Electro hydrodynamic atomization method.
Polymer used in microsponge:
• Eudragit RS-100, RSPO,S100,RL-100.
• Ethyl cellulose.
• Polystyrene.
• Acrylic polymer.
• Polyhydroxylehtylmethacrylate.
• Carbapol 934.
Eudragit RS:
Chemical formula: C19H34CINO6.
Application: Copolymer, gel, disintegrants.
Ethyl cellulose:
Other name: Ethyl ether, cellulose, E462.
Application: Emulsifier
Polystyrene:
Chemical formula: (C8H8) n
Application: Copolymer, gel
Acrylic polymer:
Application: Transparency, elasticity, light weight, copolymer
Polyhydroxylehtylmethacrylate:
Application: Copolymer, gel
Chemical formula: (C6H10O3) n
Carbapol 934:
Applications: Topical or oral
Residual solvent: Benzene
Microsponge as programmable topical delivery: Microsponge incorporates into a formulation of product such as a gel, cream, liquid or powder [41].
E.g: Benzoyl peroxide, Fluconazole Mupirocin, Hydroxyzine hcl, Terbinafine hcl, Diclofenac sodium, Hydroquinone and retinol.
The microsponge for oral delivery:
• Ketoprofen tablet of microsponge were prepared by direct compression method.
• Flurbiprofen mechanically strong tablet prepared for colon specific drug delivery.
The microsponge in delivery of biopharmaceuticals: Mainly, the fibroblast development factor was incorporated in a collagen wipe sheet it continues to release in the mouse sub cutis as the biodegradation of the sponge framework continues and its showing their action locally and its dose dependent [42-45].
Solve several issues with artificial grafts, by introducing compound a collagen microsponge with a biodegradable polymeric platform composed of polygyycolic acid with knitted mesh, reinforced on the outwards with woven poly lactic acid [46].
Release Mechanism
Study of microsponges release mechanism can be measures by;
Pressure: The active ingredient from microsponges onto skin can release by rubbing or applied the pressure [47].
Solubility: mostly the microsponges filled with water dissolvable ingredients like anti-prespirants and germicides are being releasing the component in the sight of H2O. The release mechanism is activated by diffusion taking into consideration and the partition coefficient of the component or ingredient between the microsponges and system [48-50].
Changing temperature: When ↑ skin temperature the flo w rate and release of drug is ↑.
PH triggered: Targeting or trigger the pH based release of active medicament and can be obtained by modifying the formulation.
Evaluation Methodology of Microsponge
• Determination of particle size.
• Surface topography and Morphology of microsponges.
• Determination of production yield and loading efficiency.
• True density determination.
• Characterization of pore structure.
• Compatibility studies.
• Polymer/Monomer composition.
• Resiliency.
• Dissolution profile.
• Kinetic release.
Evaluation Methods of Microsponges
Particle size determination: The analysement particle size of loaded and unloaded microsponge with the help of laser light diffractometry or by using suitable method. A graph is plotted against the cumulative percentage of drug release from microsponges of different particle size i.e. drug release study effect on particle size. The Particles having greater size than thirty micrometer can impart gritty feeling and it recommended in final topical formulation preparation the size should be b/w 25 μm-10 μm are mentioned to used.
Study of shape size configuration of microsponges: That in another way we describe as studied of “morphology of microsponge and surface topography” can be prepared microsponges by coating of gold palladium then preparation are perform by the argon atmosphere to study the surface morphology of the microsponges at room temperature then it can be further elaborated by using some of the techniques involving:
• Scanning Electron Microscopy (SEM).
• Ttransmission Electron Microscopy (TEM).
• Photon Correlation Spectroscopy (PCS) etc.
Assurances of loading efficiency and generation yield: The following equation expresses the loading efficiency (%) of the microsponges is as:

The production yield of the microsponge drug can be formulated by using the formula:
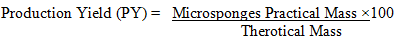
Technique used for determination of true density: Mainly applying the method of determination of true density of microsponge is calculating by using the equipped named as ultra pycnometer in which using helium gas and determined it.
Characterization of pore structure: Mercury intrusion porosimetry with the help of this methods we can easily determine the diameter of pore and volume of drug release rate. The diameter of pore of microsponge can be determine or calculated by using the Wash burn equation
D=-4γcosθ/P
Here D is diameter of pore (m); P is the pressure (psia).
Compatibility studies: The compatibility study of drug with excipients studied by;
• Thin Layer Chromatography (TLC).
• Fourier Transform Infrared spectroscopy (FT-IR).
The drug can be studied by powder (XRD) X-ray Diffraction and (DSC) Differential Scanning Colorimetry can be seen the effect of polymerization on crystallinity and with the help graph it campare. In this method take 5 mg sample of the drug is taken and measured accurately by using aluminum pan after that sealed it carefully by using heating rate at 15°C/min over a temperature range between 25°C-43°C in atmosphere of nitrogen.
Polymer/monomer composition: Factors such as drug size, drug indulges and polymer organization method helps to releasing the drug from microsphere. Polymer composition of the microsponge can be chosen according to need of formulation method it show direct activity on release rate of entrapping drug. The studies of the polymer composition can done by using/drawing the graph against drug release cumulative % vs. time.
Buoyancy: This study of resiliency of microsponges can be done to know the how beadlets is being made whether it is softer or firmer according to the demand of the final formulation or not. If there is increase of cross-linking tends it means it shows the release rate of drug is slow.
Dissolution studies: Dissolution study is done to determine the rate and extent of dug form solution it is a type of rate limiting factor, microsponges can be evaluated by application of dissolution apparatus as given in USP XXIII equipped with a modified basket contained of stainless steel with mesh size 5 μm at 37C. The speed is 150 rpm. The release mechanism is chosen according to the type of formulation i.e. topical, oral it ensure sink condition and then sample is analyzed at various time periods [51].
Kinetics of release: Kinetics release study should be helpful to understand the drug release mechanism of medication. To decide the medication discharge component and to think about the discharge profile contrasts among microsponges, the medication discharged sum versus time was utilized. The discharge information was investigated with the accompanying scientific models:
Q=k1tn or
Applying log above eq. we get
LogQ=log k1+n log t …. (1)
Here Q is indicating the amount of release at time (h),
N shows the release mechanism and
K1 is a steady constant characteristic of the drug polymer interaction.
From the incline and catch of the plot of log Q versus log t, motor parameters n and k1 were determined.
For examination purposes, the information was additionally exposed to eq. (2), which might be viewed as a straightforward, Higuchi type condition.
Q=k2t0.5+C …. (2)
Equation (2), for discharge information subject to the square foundation of time, would give a straight-line discharge profile, with k2 displayed as a root time disintegration rate steady and C as a consistent (Tables 1-5).
| S.No | Category | Excipients |
|---|---|---|
| 1 | Analgesic | Trolamine |
| 2 | Antibiotic | Erythromycin |
| 3 | Anti-bacterial | Mupirocin |
| 4 | Anti-acne | Benzoyl peroxide |
| 5 | Anti-diabetic-ii | Mitiglinide calcium |
| 6 | Anti-emetic | Domperidone, ondansetron |
| 7 | Anti-gout | Allupurinol |
| 8 | Anti glucoma | Acetazolamide |
| 9 | Anti-hypertensive | Candesratan clexitel, metoprolol succinate |
| 10 | Anti-histamine | Hydroxize hcl |
| 11 | Anthelmintic | Albendazole |
| 12 | Anti hyperlipidmic | Atrovastatin |
| 13 | Anti-viral | Acyclovir, ritonavir |
| 14 | Anti-inflammatory | Curcumin, betamethasone, mesalamine |
| 15 | Arthritis | Diclofenac, diethylamine, piroxicam |
| 16 | Anti-fungal | Fluconazol, iticonazole, miconazole, bifluconazole, sertaconazolenitreate, tolnaflote |
| 17 | Antiulcer | Famotidine |
| 18 | Control sleeping problem | Melatonin |
| 19 | Cns stimulant | Bupropion |
| 20 | Colon rectal cancer | 5-fluro uracil |
| 21 | Corticosteroids | Flucinoloneacetonide, mometasone furoate, predinosolone |
| 22 | Dermatosis | Miconazo |
| 23 | K+channel opener | Micorandil |
| 24 | Nsaids | Pcm,ibrufen, indomethacin, lornoxicam, ketoprofen, acelofenac, celecoxib, diclofenac sodium |
| 25 | Proton pump inhibitor | Lansoperozole |
| 26 | Sun screen agent | Oxybenzone |
| 27 | Schizorphenia | Resperidone |
| 28 | Vitamin a | Retinol |
Table 1: Drugs used for microsponge are as follows.
| Category | Exicepents used |
|---|---|
| Cream | Hydroquinone and retinol |
| Gels | Benzoyl peroxide, acyclovir, fluconazole, mupricone, hydroxyzin hcl, terbinafine Hcl, declofenac sodium |
| Grafts | Poly (lactic-co-glycolic acid) |
| Injection | Basic fibroblast growth factor |
| Implant | Poly (dl-lactic-co-glycolyyic acid) |
| Lotion | Benzoyl per oxide |
| Tablet | Pcm.chloropheraminemaleate, ketoprofen, fenofibrate, flubprofen, dicyclomine, meloxicam |
| Microencapsulation | Babachi oil |
Table 2: Microsponge preparation in different forms.
| S.No. | Application | Advantages |
|---|---|---|
| 1 | Stmscreens | Long lasting product efficacy, with improved protection against sunbums and sun related injmies even at elevated concentration and with reduced irritancy and sensitization |
| 2 | Anti-acne e.g. Benzoylperoxide |
Maintained efficacy with decreased skin iffitation and sensitization. |
| 3 | Anti-inflammatory e.g.hydrocortisone |
Long lasting activity with reduction of skin allergic response and denuatoses. |
| 4 | Anti-dandn1ffs e.g.zinc pyrithione, selenium sulfide |
Reduced tmpleasant odom with lowered iffitation with extended safety and efficacy. |
| 5 | Antipruritics | Extended and improved activity. |
| 6 | Skin depigmenting agents e.g. hydroquinone |
Improved stabilization against oxidation with improved efficacy and aesthetic appeal. |
Table 3: Applications of micro sponges with respect to their advantages.
| Patent no | lnvento1·s | Publication Date |
|---|---|---|
| US4690825 | Won, Richard | 1987 |
| US4863856 | Dean RC Jr et al. | 1989 |
| US5292512 | Schaefer et al | 1989 |
| US5135740 | Katz et al. | 1992 |
| US5679374 | Fanchon; Chantal et al | 1994 |
| US5316774 | Eury, Robert P et al. | 1994 |
| US5725869 | Lo; Ray J.R. | 1996 |
| US6395300 | Straub et al. | 1999 |
| US6211250 | Tomlinson et al | 2001 |
| US20030232091 | Shefer et al. | 2002 |
Table 4: Patents filled on microsponge.
| S.No | Category | Drug | Monomer |
|---|---|---|---|
| 1 | Antibiotic | Erythromycin | Ethyl cellulose |
| 2 | Anti-diabeticii | Mitiglinide calcium | Ethylcellulose, eudragit rs -100 |
| 3 | Anti-emetic | Domperidone | Eudragit rs 100 |
| 4 | Anti-gout | Allupurinol | Eudragit epo, ethylcellulose |
| 5 | Anti glucoma | Acetazolamide | Pluronicf-127, ethyl cellulose |
| 6 | Anti-hypertensive | Candesratan clexitel | Eudragit rs-100, eudragit rl-100 |
| 7 | Anthelmintic | Albendazole | Eudragit rs-100 |
| 8 | Anti hyperlipidmic | Atrovastatin | Eudragit rs-100, carbopol934 |
| 9 | Anti-viral | Acyclovir | Ethylcellulose, carbapol |
| 10 | Anti-inflammatory | Curcumin | Ethylcellulose |
| 11 | Anti-fungal | Econazole | Ethylcellulose, carbopol-934, eudragit rs-100 |
| 12 | Antiulcer | Famotidine | Hpmc, eudragit-100 |
| 13 | Cns stimulant | Bupropion | Ethylcellulose |
| 14 | Colon rectal cancer | 5-fluro uracil | Eudragit rs-100, l100, s100 |
| 15 | Corticosteroids | Mometasone furoate | Eudragitrs-100 |
| 16 | K+channel opener | Micorandil | Eudragits-100, rspo, rlpo |
| 17 | Proton pump inhibitor | Lansoperozole | Eudragit s-100, l-100 |
| 18 | Sun screen agent | Oxybenzone | Ethylcellulose |
Table 5: Monomers used in microsponges.
Monomers and Drugs Used In the Categorywise Preparation of Microsponges as per Research and their Analysis.
Antibiotic: Preparation of antibiotic microsponge gel of erythromycin by using the monomers like ethyl cellulose and that result will shows the extend release of gel upto 8 hour also indicating in in his research that its reduced its side effects [47].
Anti-diabetic-ii: Preparation of gastro retentive microsponge of mitiglinide calcium it is a type-2 diabetes, by using monomers in their preparation of msp was Ethylcellulose, eudragit rs-100 that reascercher has to be found that the prolonging action of mitiglinide calcium with lowering the frequency of dosing so that it would be convenient to the patient.
Anti-emetic: Preparation of microsponge loaded capsuled of antiemetic drug of domperidone using the monomer of eudragit rs 100 after making the msp capsule of domperidone reascercher should analysed that improved in bioavailabity of microsponge loaded capsule of domperidone with prolonging the drug release.
Anti-gout: Antigout preparation of microsponge of drug is allopurinol floating MSP with suitable monomers eudragit EPO, ethylcellulose, the reascercher has to be found in her result the drug release rate is upto 12 hr.
Anti glucoma: Microsponge preparation of acetazolamide insitu gel by using monomer in her preparation of pluronicf-127, ethyl cellulose, that’s improved the therapeutic efficacy of acetazolamide gel after being preparing of microsponge gel.
Anti-hypertensive: Metoprolol succinate preparing colon specific microsponge tablet using monomer of ethylcellulose that show the micro flora activating sustaining release of microsponge tablet of Metoprolol succinate in colonic system that show because of the microsponge having the property.
The second drug of antihypertension is candesratan clexitel using different monomer in her study as comparison to other drug of antihypertension that’s name was eudragit rs-100, eudragit rl-100 in the study of candesartan clexitel reascercher has to be found that dissolution rate should be improved after being preparation of microsponge and also improved its therapeutic efficacy.
Anthelmintic: It is a different category albendazole preparation of microsponge by using Monomers of eudragit rs-100 by preparing its microsponge its shows that having sustaining release property towards the tissue parasite in the research.
Anti hyperlipidmic: Preparing a wound heals emu gel of microsponge by using the drug of atorvastatin monomers of eudragit rs-100, carbopol934 it found that the wound gel preparation of microsponge have great effect on ulcer wound and quick acting.
Anti-viral: Acyclovir microsponge topical gel preparation through using of monomers ethylcellulose, carbapol leads to sustained release action.
2nd drug of preparation of antiviral category is ritonavir preparing the microsponge topical gel by using same polymer but result is different in this research show the improvement in oral bioavailabity of drug after preparing the MSP.
Anti-inflammatory: Microsponge capsule is prepared by encapsulated drug of curcumin using monomers of ethylcellulose, curcumin itself has several characteristic so by preparing the microsponge capsule of curcumin improved the bioavailabity and drug release of curcumin, curcumin taste is not so good so by being preparing the microsponge it would helpful in taste masking.
Anti-fungal: Topical microsponge hydrogel of Econazole Nitrate with the help of monomer like ethylcellulose, carbopol-934, eudragit rs-100 that’s result indicating the sustained releasing of drug. Serataconazole nitrate is belong to the antifungal category preparation of topical MSP monomer using the eudragit RS 100, carbopol 934 that’s results has to be found that improvement of drug efficacy. Tolnaftate is Antifungal drug preparation of microsponge topical gel using the eudragit RL 100, RS 100 researcher has to be found in has studied that after preparation of microsponge of tolfonate gel has the improving drug release. [48-51]
Antiulcer: Famotidine floating microsponge preparation HPMC, eudragit-100 use these microsponge reascercher has to be found that prolonging release of drug.
CNS stimulant: Drug preparation of floating microsponge of bupropion belonging to the CNS stimulation category that’s shows the improved bioavailabity and controlled release of microsponge by helping of monomers ethylcellulose.
Colon rectal cancer: 5-fluro uracil microsponge capsule of enteric coated HPMC 5-flurouracil capsule treat the colon rectal cancer by polymer eudragit rs-100, L100, s100. Thats study helped in stimuli responsive drug release in research.
Corticosteroids: Topical microsponge of mometasone furoate to treat psoriasis monomers eudragitrs-100 using preparation in the research reascercher has to be found that less sideffect with controlled release.
K+ channel opener: Microsponge sustained release tablet preparation of nicorandil used of monomers, eudragits-100, rspo, rlpo it controlled the release of drug in the form of microsponge upto 24 hr.
NSAIDS (Non-Steroidal Anti-Inflammatory Drug): Acelofenac preparation of microsponge gel using the monomer of eudragit RS 100, ethylcellulose that’s results after preparing the microsponge gel of acelofenac drug it showing the prolonging action upto 9 hr.
• Diclofenac sodium belongs to the NSAIDS category that is another preparation of microsponge. Micro sponge’s colon targeted preparation of diclofenac sodium using in the preparation of suitable monomers and preparation of quasi emulsion method that result found enhancing in bioavailabity.
• Indomethacin preparation of microsponge it is one of another drugs of NSAIDS that is usually prepared by microsponge by including the preparation of monomers of eudragit RS 100 that’s results in the research showing that managing the controlled drug release.
• Ketoprofen is the one of category of NSAID (Arthritis) used monomer of Eudragit RS 100 Enhancing medicament release with increasing bioavailability.
• Lornoxicam one of the researchers also used another drug from NSAID (rheumatoid arthritis) that is prepared by MSP tablets for colon targeted used the monomer of ethyl cellulose that in her research found that specific targeting the colonic target.
• Piroxicam also belong to the NSAID (arthritis) that was being used for the preparation of microsponge topical gel for transdermal delivery using the monomer in their preparation eudragit RS 100, S 100, RL 100, carbapol 934 after being preparing the microsponge gel result has to be found that improving the dissolution and drug release with reducing side effects, good therapeutic efficacy.
Proton pump inhibitor: Lansoperozole preparation of microsponge avoiding the degradation of acidic medium using with suitable monomer of eudragit s-100, L-100.
Sun screen agent: Oxybenzone gel of microsponge method of preparation is quasi emulsion method using suitable monomer of Ethylcellulose reduced the irritaton and prolonging action (Table 6).
| Product name | Pharmaceutical uses | infanufacturer |
|---|---|---|
| Glycolic acid moistwizer w/SPF 15 |
Anti-wrinkle s, soothing | AMCOL health and beauty solution |
| Retin A micro | Acne-vulgaris | Oztho-McNeil pharmaceutic.al, Inc. |
| Carac cream, 0.5% | Actinic keratoses | Dermik laboratories, Inc. |
| Llne eliminator dual retinol facial treatment |
Anti-wrinkle | Avon |
| Retinol 15 night cream | Anti-wrinkle s | Sothys |
| Retinol cream | Helps maintain healthy skin | Biomedic |
| EpiQuin Micro | Hyper pigmentation | SkinMedica Inc |
| Sports cream RS and XS | Anti-inflammatory | Embil pharmaceutical Co.Ltd. |
| Salicylic peel 20 | Excellent exfoliation | Biophora |
| Oil freematte block SPF 20 | Swiscreen | Dermalogica |
| Lactrex TM 12% moisturizing cream |
Moistwizer | SDR pharmaceuticals, Inc |
| Dermalogica oil control lotion | Skin protectant | John and ginger dermalogica skin care products |
| Ultra guard | Protectsbabvns skin | Scott paner comnanv |
Table 6: List of marketed products based on micro sponge.
With demand for modern and distinctly green pharmaceutical in addition to cosmetic merchandise, the market place holds vast capacity for microsponge technology and the flexibility they offer. As formulators remember new and creative approaches to supply actives, they are able to understand the total capabilities of those specific substances offering more advantageous safety, stepped forward stability, reduced facet results from actives, more suitable multifuntionality and advanced component compatibility. Complemented by novel development tactics and innovative formulation strategies, microsponge transport device may be a winning method for a new era of pharmaceutical and cosmetic industry. Microsponges have an awesome benefit over the present traditional topical dosage forms for the remedy of tropical illnesses; it's far a completely unique generation for the controlled launch of topical agents additionally use for oral in addition to biopharmaceutical drug transport. This indicates high quality over other products by using non-mutagenic, non-toxic and non-irritant. At lasts not least there is huge work to be done in microsponge after analyzing the research on microsponge it would be proved that it improved the drug stability, efficacy, bioavailabity and several other advantages in the form of microsponge and also show several unknowns facts and result has to be come in the research it have a selfsterilizing like property so by using its property we can concluded so many new innovation and new drug delivery system because it was itself a well stabilizer as compare to the other forms of drugs delivery system.
The authors are grateful to MM (deemed to be university), Mullana, Ambala for providing facility and helping in completing this review article.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Googlescholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Chauahan R (2023) One Step Successor towards Drug Delivery System: Microsponge. Act Psycho. 9:7198
Copyright: © 2023 Chauahan R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.